|
Bloodborne
Pathogens
After
Mass-Casualty
Events
August
8, 2008
Noting
that
health
authorities
in
Israel
and
London
have
reported
hepatitis
B-infected
tissue
and bone
fragments
were
found
after
suicide
bombings
and
could
have
infected
survivors
and
rescuers,
the
Centers
for
Disease
Control
and
Prevention
has
issued
new
guidance
for
using
immunizations
and
post-exposure
prophylaxis
for
tetanus
and
bloodborne
pathogens
(including
HBV,
hepatitis
C, and
HIV) in
people
wounded
in
bombings
or other
mass-casualty
events,
whether
explosions
or
natural
disasters.
The
recommendations
were
published
jointly
in CDC's
Morbidity
and
Mortality
Weekly
Report
on Aug.
1 and in
the
American
Medical
Association's
Disaster
Medicine
and
Public
Health
Preparedness
journal.
CDC said
the
recommendations
represent
the
consensus
of U.S.
federal
public
health
officials
and
reflect
the
experience
and
input of
public
health
officials
at all
levels
of
government
and the
acute
injury
response
community.
They are
available
at
www.cdc.gov/MMWR/preview/mmwrhtml/rr5706a1.htm
and at
www.dmphp.org
Louisa
E.
Chapman
of the
Immunization
Services
Division
in CDC's
National
Center
for
Immunizations
and
Respiratory
Diseases
and her
co-authors
assessed
the
infection
risk for
each
hazard
and for
the most
part
recommend
no
action
is
needed
to
prevent
infections.
However,
they
recommend
testing
for HCV
when an
HCV-infected
source
"is
known or
thought
to be
likely
on the
basis of
the
setting
in which
the
injury
occurred
or
exposure
to blood
or
biologic
material
from a
bomber
or
multiple
other
injured
persons
is
suspected."
A
decision
to
perform
testing
of
specific
persons
might be
based on
the
judgment
of the
treating
physician
and the
preferences
of the
individual
patient;
testing
during a
follow-up
referral
might be
a more
feasible
logistical
option
in the
setting
of
response
to a
mass-casualty
event,
they
concluded.
The
guidance
says
health
care
providers
should
determine
appropriate
actions
in
response
to
evaluation
of
casualties
of
bombings
or other
mass-casualty
events,
assessing
individual
exposure
risk by
categorizing
the
patient
into one
of three
exposure
risk
categories
and
assigning
each
person
to the
highest
risk
category
for
which he
or she
qualifies.
"When
evaluating
management
choices
for
casualties
of
bombings
or other
mass-casualty
events,
health-care
providers
should
assume
that
exposure
to blood
from
other
injured
persons
is
likely
unless
available
information
on the
circumstances
of
injury
suggests
otherwise.
Blast
injuries
result
occasionally
in
traumatic
implantation
of bone
or other
biologic
material
that is
alien to
the
wounded
person.
Testing
of such
matter
is not
recommended
as a
useful
adjunct
for
clinical
management
of
wounded
persons.
Public
health
authorities
can
provide
assistance
in
assessing
exposure
risk for
affected
groups
of
injured
persons,"
it
states.
|

Recommendations
and
Reports |
 |
 |
|
August
1, 2008
/
57(RR06);1-19 |
Recommendations
for Postexposure
Interventions to
Prevent
Infection with
Hepatitis B
Virus, Hepatitis
C Virus, or
Human
Immunodeficiency
Virus, and
Tetanus in
Persons Wounded
During Bombings
and Other
Mass-Casualty
Events ---
United States,
2008
Recommendations
of the Centers
for Disease
Control and
Prevention (CDC)
Summary
This
report outlines
recommendations
for postexposure
interventions to
prevent
infection with
hepatitis B
virus, hepatitis
C virus, or
human
immunodeficiency
virus, and
tetanus in
persons wounded
during bombings
or other events
resulting in
mass casualties.
Persons wounded
during such
events or in
conjunction with
the resulting
emergency
response might
be exposed to
blood, body
fluids, or
tissue from
other injured
persons and thus
be at risk for
bloodborne
infections. This
report adapts
existing general
recommendations
on the use of
immunization and
postexposure
prophylaxis for
tetanus and for
occupational and
nonoccupational
exposures to
bloodborne
pathogens to the
specific
situation of a
mass-casualty
event. Decisions
regarding the
implementation
of prophylaxis
are complex, and
drawing
parallels from
existing
guidelines is
difficult. For
any prophylactic
intervention to
be implemented
effectively,
guidance must be
simple,
straightforward,
and logistically
undemanding.
Critical review
during
development of
this guidance
was provided by
representatives
of the National
Association of
County and City
Health
Officials, the
Council of State
and Territorial
Epidemiologists,
and
representatives
of the acute
injury care,
trauma and
emergency
response medical
communities
participating in
CDC's Terrorism
Injuries:
Information,
Dissemination
and Exchange (TIIDE)
project. The
recommendations
contained in
this report
represent the
consensus of
U.S. federal
public health
officials and
reflect the
experience and
input of public
health officials
at all levels of
government and
the acute injury
response
community.
Introduction
Public health
authorities must
consider how to
provide care to
injured persons
in the event of
acts such as
bombings that
result in mass
casualties.
During
1980--2005, of
318 acts of
terrorism
investigated by
the Federal
Bureau of
Investigation
(FBI) in the
United States or
territories, 208
(65%) involved
attempted
bombings; of
these 208
attempts, 183
(88%) succeeded.
The majority of
these acts were
committed by
domestic
extremist groups
that
intentionally
targeted
property and did
not cause deaths
or injuries in
persons;
however, 19
bombings (10% of
those that were
successful)
resulted in 181
deaths and 1,967
injured
survivors. These
figures do not
include
mass-casualty
incidents that
occurred outside
the United
States and its
territories or
those that
occurred on U.S.
soil that were
classified as
crimes,
accidents,
unintended
negligence, or
terrorist
incidents other
than bombings
(e.g., the 2,972
persons killed
as a result of
the terrorist
attacks of
September 11,
2001). A total
of 1,967 (91%)
persons injured
during terrorist
bombings in the
United States
and
approximately
12,000 (80%)
persons injured
during the
terrorist
attacks of
September 11,
2001, survived (1).
Military
health-care
providers
frequently must
respond to
mass-casualty
events. During
October 7,
2001--March 1,
2008, of 35,630
casualties
incurred by U.S.
Department of
Defense forces
involved in
Operation
Enduring Freedom
(OEF) in
Afghanistan and
Operation Iraqi
Freedom in Iraq
(OIF), 27,441
(77%)
resulted from
mass-casualty
events.
Explosive
devices
accounted for
23,277 (65%) of
these
casualties. Of
27,441 persons
wounded during
OEF- and OIF-related
mass-casualty
events, 24,433
(89%) survived
(U.S. Department
of Defense,
unpublished
data, 2008).
In August
2001, the
Israeli health
ministry
announced that
tissue from two
suicide bombers
had tested
positive for
evidence of
hepatitis B
virus (HBV) (2).
A 2002 case
report from
Israel described
evidence of
hepatitis B
virus in a bone
fragment that
had
traumatically
implanted into a
bombing survivor
(3).
Traumatically
implanted bone
fragments
removed from
five survivors
of the 2005
London bombings
were taken
directly to
forensic custody
without testing
for bloodborne
pathogens (4)
These
observations
support the
potential for
explosions to
result in
transmission of
infections among
persons injured
during the event
and indicate
that emergency
responders and
health-care
providers in the
United States
need uniform
guidance on
prophylactic
interventions
appropriate for
persons injured
in bombings and
other events
resulting in
mass casualties.
Wounds resulting
from
mass-casualty
events require
the same
considerations
for management
as similar
injuries
resulting from
trauma cases not
involving mass
casualties,
including the
risk for
tetanus. In
addition,
exposure of
wounds, abraded
skin, or mucous
membranes to
blood, body
fluids, or
tissue from
other injured
persons
(including
suicide bombers
and bombing
casualties)
might carry a
risk for
infection with a
bloodborne
virus. Injured
survivors of
mass-casualty
events are at
risk for
infection with
HBV, hepatitis C
virus (HCV), or
human
immunodeficiency
virus (HIV) and
for tetanus.
Decisions
regarding the
administration
of prophylaxis
after a
mass-casualty
event are
complex, and
drawing direct
parallels from
existing
guidelines
regarding
prophylaxis
against
bloodborne
pathogens in
occupational or
nonoccupational
settings is
difficult.
Assessment of
risk factors
commonly used to
estimate the
need for
prophylactic
intervention
might not be
possible in the
setting of
response to a
mass-casualty
event because
responses to
such events
might overwhelm
local emergency
response
facilities, and
medical response
staff will be
focused
primarily on
rendering
lifesaving
trauma
treatments.
Because no
uniform guidance
existed for
posstexposure
interventions to
prevent
bloodborne
infections and
tetanus among
U.S. civilians
or military
personnel
wounded during
mass-casualty
event, CDC
convened a
Working Group
comprising
experts in
injury response,
immunizations,
bloodborne
infections,
tetanus, and
federal-,
state-, and
local-level
public health
response to
develop such
guidance.
The
recommendations
in this report
pertain only to
bombings and
other
mass-casualty
events and are
not meant to
supplant
existing
recommendations
for other
settings. In a
situation
involving a
substantial
number of
casualties, the
ability to
assess medical
and vaccination
histories or the
risks associated
with the source
of exposures
might be
limited, as
might the supply
of biologics.
For this reason,
in certain
instances, the
recommendations
provided in this
report differ
from standard
published
recommendations
for vaccination
and prophylaxis
in other
settings. These
recommendations
are not meant to
supplant
existing
recommendations
for other
settings and
apply only to
the specific
situation of an
event involving
mass casualties.
In addition, the
recommendations
provided in this
report are
limited to
issues regarding
initial
postexposure
management for
bloodborne
pathogens and
tetanus
prophylaxis.
Other
prophylactic
measures that
might be
appropriate
(e.g., use of
antibiotics for
the prevention
of bacterial
infection) are
not discussed in
this report.
Federal law
requires the use
of a Vaccine
Information
Statement (VIS)
before the
administration
of vaccines
against HBV or
tetanus. VIS
forms are
available at
http://www.cdc.gov/vaccines/pubs/vis/default.htm.
Whenever
feasible, a VIS
form should be
provided to
patients or
guardians before
vaccination.
Individual
states set forth
their own legal
requirements as
to what
constitutes the
nature of
informed consent
that might be
required before
certain medical
interventions.
In general,
these statutes
also provide for
exemptions in
emergency
circumstances.
It is these
state-specific
laws that should
guide response
when informed
consent would be
applicable, but
the
circumstances of
response to a
mass-casualty
event might
preclude
adherence to
standard
informed consent
processes.
Emergency
responders and
health-care
providers should
consult with
their legal
counsel for
guidance
regarding the
relevant laws of
their
jurisdictions in
advance of any
mass-casualty
event.
Methods
This report
was developed
through
consultation
among persons
with expertise
in immunization
and other
prophylactic
interventions
against
bloodborne and
other
infections,
physicians who
specialize in
acute
injury-care
medicine (trauma
and emergency
medicine), and
local, state,
and federal
public health
epidemiologists.
Thus, the
recommendations
in this report
represent the
best consensus
judgment of
expert opinion.
This report
adapts existing
recommendations
on the use of
immunization and
postexposure
prophylaxis in
response to
occupational and
nonoccupational
exposures to
bloodborne
pathogens in the
United States to
the specific
mass-casualty
event setting
while
acknowledging
the difficulty
of drawing
direct
parallels. This
adaptation also
draws on
guidance and
practices
developed
previously and
in use in the
United Kingdom
and Israel (2,5--7).
These
recommendations
were adopted
through a
process of
expert
consultation and
consensus
development.
First, CDC
drafted proposed
preliminary
recommendations
on the basis of
relevant
existing U.S.
guidance and
practices of
Israel and the
United Kingdom (2,5--7).
These proposed
recommendations
were discussed
by
representatives
of the U.S. and
international
trauma response
community at a
May 2006 meeting
in Atlanta,
Georgia;
following this
discussion, the
initial draft
was revised. A
working group
then was
convened
comprising CDC
staff members
with expertise
in injury
response,
tetanus, viral
hepatitis, HIV
infection,
immunization and
postexposure
prophylaxis, and
occupational
safety and
health, and
representatives
of the National
Association of
County and City
Health Officials
and the Council
of State and
Territorial
Epidemiologists
with experience
in local and
state level
public health
response. This
group worked
through the
draft section by
section to
revise, update,
and refine the
recommendations;
this revised
document was
shared again
with
representatives
of the U.S. and
international
trauma response
community for
additional
comment during a
meeting in
Atlanta,
Georgia, in
August 2007.
Because this
guidance met the
requirements
established by
the Office of
Management and
Budget (OMB) for
a Highly
Influential
Scientific
Assessment
(HISA)
(available at
http://www.whitehouse.gov/omb/memoranda/fy2005/m05-03.html),
the
recommendations
underwent a
final process of
external review
in addition to
undergoing
internal CDC
review for
scientific
content. As part
of the OMB HISA
peer review, the
document was
posted on CDC's
website for
public comment.
An external
expert panel
subsequently
reviewed and
critiqued the
document, the
public comments,
and CDC's
response to
those comments,
and the document
was revised a
final time in
response to the
external review
process.
Bloodborne
Pathogens of
Immediate
Concern
Although
transfusions and
injuries from
sharp objects
(e.g.,
needlestick)
have been
associated with
the transmission
of multiple
different
pathogens (8,9),
three bloodborne
pathogens merit
specific
consideration in
mass-casualty
situations: HBV,
HCV, and HIV.
All three
viruses are
endemic at low
levels in the
United States
and can be
transmitted by
exposure of
infectious blood
to an open wound
or, more rarely,
to skin
abrasions or
through exposure
to intact mucous
membranes. These
viruses also can
be transmitted
by similar
exposures to
other body
fluids or
tissues from
infected
persons.
Infection risks
and options for
postexposure
prophylaxis
vary, depending
on the virus and
the type of
injury and
exposure.
Because
hepatitis A
virus (HAV) is
transmitted via
the fecal-oral
route and is not
considered a
bloodborne
pathogen (10),
HAV prophylaxis
is not
recommended
during a
mass-casualty
event.
The
information
typically used
in occupational
settings to
guide
prophylactic
intervention
decisions
(including the
circumstances of
the injury,
background
prevalence of
disease, or risk
for infection of
the source of
exposure) might
not be as
clearly
interpretable or
as readily
available in a
mass-casualty
setting. For
example, both
the extent of
exposed
disrupted skin
and the volume
of blood
contributing to
the exposure
might greatly
exceed that of
usual
occupational
exposures. In
addition,
injured persons
might be exposed
to blood from
multiple other
persons or to
biologic
material from
the body of a
bomber or
another injured
person. The HBV,
HCV, and HIV
status of the
source(s)
usually will be
unknown, and
timely
ascertainment
might not be
practical. If
the circumstance
in which each
victim was
injured can be
characterized,
this information
can be used to
assess the
likelihood that
an injured
person was
exposed to
another person's
blood. However,
when such
information is
not readily
available for
persons injured
during
blast-related
mass-casualty
events, such
blood exposure
should be
assumed.
Hepatitis
B Virus
The prevalence
of chronic HBV
infection in the
United States is
approximately
0.4%. Prevalence
varies by race,
ethnicity, age
group,
geographic
location, and
individual
history of risk
behaviors (11).
Newly
acquired HBV
infection often
is asymptomatic;
only 30%--50% of
children aged >5
years and adults
have initial
clinical signs
or symptoms (11).
The fatality
rate among
persons with
reported cases
of acute
symptomatic
hepatitis B is
0.5%--1.0% (11).
No specific
treatment exists
for acute
hepatitis B.
Acute hepatitis
B infection
fails to resolve
and instead
progresses to
chronic HBV
infection in
approximately
90% of those
infected as
infants, 30% of
children
infected at age
<5 years, and
<5% of persons
infected at age
>5 years
(11).
Overall,
approximately
25% of persons
who become
chronically
infected during
childhood and
15% of those who
become
chronically
infected after
childhood die
prematurely from
cirrhosis or
liver cancer (11).
Therapeutic
agents approved
by the U.S. Food
and Drug
Administration
(FDA) for
treating chronic
hepatitis B can
achieve
sustained
suppression of
HBV replication
and remission of
liver disease
for certain
persons (11).
HBV is
transmitted by
percutaneous or
mucosal exposure
to infectious
blood or body
fluids. Although
hepatitis B
surface antigen
(HBsAg) has been
detected in
multiple body
fluids, only
serum, semen,
and saliva have
been
demonstrated to
be infectious (11).
Serum has the
highest
concentration of
HBV, with lower
concentrations
in semen and
saliva. HBV
remains viable
for 7 days or
longer on
environmental
surfaces at room
temperature (11).
Among
susceptible
health-care
personnel, the
risk for HBV
infection after
a needlestick
injury involving
an HBV-positive
source is
23%--62% (12).
Prompt and
appropriate
postexposure
prophylaxis
(PEP)
intervention
reduces this
risk. Many
infections that
occurred before
widespread
vaccination of
health-care
personnel
probably
resulted from
unapparent
exposures (e.g.,
inoculation into
cutaneous
scratches,
lesions, or
mucosal
surfaces) (12).
Both
passive-active
PEP with
hepatitis B
immune globulin
(HBIG) combined
with hepatitis B
vaccine and
active PEP with
hepatitis B
vaccine alone
have been
demonstrated to
be highly
effective in
preventing
transmission
after exposure
to HBV (12).
HBIG alone has
been
demonstrated to
be effective in
preventing HBV
transmission.
However, since
hepatitis B
vaccine became
available, HBIG
is used
typically (and
preferentially)
as an adjunct to
vaccination (11).
The major
determinant of
effectiveness of
PEP is early
administration
of the initial
dose of vaccine
(or HBIG). The
effectiveness of
PEP diminishes
the longer after
exposure it is
initiated (12).
Studies are
limited on the
maximum interval
after exposure
during which PEP
is effective,
but the interval
is unlikely to
exceed 7 days
for perinatal
and needlestick
exposures (12).
No data are
available on the
efficacy of
HBsAg-containing
combination
vaccines when
used to complete
the vaccine
series for PEP,
but the efficacy
of combination
vaccines is
expected to be
similar to that
of
single-antigen
vaccines because
the HBsAg
component
induces a
comparable
antibody
response (12).
Antiviral PEP is
not available
for HBV.
A policy of
liberal use of
hepatitis B
vaccine for PEP
after bombings
or in other
mass-casualty
situations is
recommended
because of the
high
concentration of
HBV in blood of
infected
persons, the
durability of
HBV in the
environment, and
the efficacy and
relative ease of
administration
of vaccine (11).
Such use is
consistent with
existing
recommendations
for
administering
the hepatitis B
vaccine series
as PEP for
persons (e.g.,
health-care
personnel or
sexual assault
victims) exposed
to a source with
unknown HBV
infection status
(11,12).
In general, PEP
for HBV will be
warranted for
previously
unvaccinated
persons if
wounds,
nonintact skin,
or intact mucous
membranes might
have been
exposed to blood
or body fluids
from another
person or
persons. In a
mass-casualty
setting, failure
to provide
hepatitis B
vaccination when
needed could
result in
preventable
illness, whereas
unnecessary
vaccination is
unlikely to
cause harm (11).
Completion of
primary
vaccination at
the time of
discharge or
during follow-up
visits should be
ensured for all
persons who
receive an
initial
hepatitis B
vaccine dose as
part of the
acute response
to a
mass-casualty
event.
If hepatitis
B vaccine is in
short supply,
assessing how
likely a person
is to have been
vaccinated
previously might
be necessary. In
general,
hepatitis B
vaccination
rates are
highest among
children aged
<17 years
(80%--90%) and
health-care
personnel
(approximately
80%) (Table
1) (13--15)
(see
Pathogen-Specific
Management
Recommendations).
Hepatitis
C Virus
The prevalence
of chronic HCV
infection in the
United States is
approximately
1.3% (16).
Prevalence
varies by
race/ethnicity,
age group,
geographic
location, and
individual
history of risk
behaviors (16,17).
Persons with
acute HCV
infection
typically either
are asymptomatic
or have a mild
clinical
illness.
Antibody to HCV
(anti-HCV) can
be detected in
80% of patients
within 15 weeks
after exposure
and in 97% of
patients by 6
months after
exposure.
Chronic HCV
infection
develops in
75%--85% of
infected
persons. The
majority remain
asymptomatic
until onset of
cirrhosis or
end-stage liver
disease, which
develops within
20--30 years in
approximately
10%--20% of
infected persons
(17).
HCV is
transmitted
primarily
through exposure
to large amounts
of blood or
repeated direct
percutaneous
exposures to
blood (i.e.,
transfusion or
injection-drug
use). HCV is not
transmitted
efficiently
through
occupational
exposures to
blood; the
average
incidence of
anti-HCV
seroconversion
after accidental
percutaneous
exposure from an
HCV-positive
source is 1.8%
(range: 0--7%),
with one study
indicating that
transmission
occurred only
from hollow-bore
needles (17).
Transmission
rarely occurs
through mucous
membrane
exposures to
blood, and in
only one
instance was
transmission in
a health-care
provider
attributed to
exposure of
nonintact skin
to blood (18).
The risk for
transmission
from exposure to
fluids or
tissues other
than
HCV-infected
blood has not
been quantified
but is expected
to be low. The
exact duration
of HCV viability
in the
environment is
unknown but is
at least 16--23
hours (19,20).
Immune
globulin and
antiviral agents
are not
recommended for
PEP after
exposure to
HCV-positive
blood. No
vaccine against
HCV exists. In
the absence of
PEP for HCV,
recommendations
for postexposure
management are
intended to
achieve early
identification
of infection
and, if present,
referral for
evaluation of
treatment
options. No
guidelines exist
for
administration
of therapy
during the acute
phase of HCV
infection.
However, limited
data indicate
that antiviral
therapy might be
beneficial when
started early in
the course of
HCV infection.
When HCV
seroconversion
is identified
early, the
person should be
referred for
medical
management to a
knowledgeable
specialist (12,17).
Testing is
not routinely
recommended in
the absence of a
risk factor for
infection or a
known exposure
to an
HCV-positive
source (17).
However, current
public health
practice often
does include
advising testing
for potential
exposures to
unknown sources
(e.g.,
playground
incidents
involving
needlestick or
health-care
exposures
involving
possible needle
or syringe reuse
or inadequately
disinfected
equipment). In
the setting of a
bombing or other
mass-casualty
event, both the
extent of
exposed
disrupted skin
and the volume
of blood
contributing to
the exposure
might greatly
exceed that of
usual
occupational
exposures. Thus,
baseline and
follow-up HCV
testing should
be considered
for persons
injured during
bombings or
other
mass-casualty
events whose
penetrating
injuries or
nonintact skin
are suspected to
have come into
contact with
another person's
blood or body
fluids (see
Pathogen-Specific
Management
Recommendations).
Human
Immunodeficiency
Virus
The overall
prevalence of
HIV infection in
the United
States was
estimated to be
311.5 per
100,000
population
(0.31%) in 2005,
with wide
geographic
variability
(range: 26.4 per
100,000
population
[0.03%] [North
Dakota]--2,060
per 100,000
population
[2.06%]
[Washington,
DC]) (21).
Prevalence might
vary greatly
among
subpopulations
within the same
communities
(e.g., residents
of a nursing
home compared
with residents
of transitional
housing
associated with
a drug treatment
program). The
principal means
of transmission
in the United
States is
through sexual
contact or
through sharing
of
injection-drug
use equipment
with an infected
person (21).
Exposures also
occur in
occupational
settings
(principally
among
health-care
personnel) and
infrequently can
result in
transmission.
Guidelines for
the use of
antiretroviral
PEP in both
occupational and
nonoccupational
settings have
been published
previously (22--24),
but these
documents do not
specifically
address
situations
involving mass
casualties.
Potentially
infectious
materials
include blood
and visibly
bloody body
fluids, semen,
and vaginal
secretions.
Cerebrospinal
fluid, synovial
fluid, pleural
fluid,
peritoneal
fluid,
pericardial
fluid, and
amniotic fluid
also are
considered
infectious, but
the transmission
risk associated
with them is
less well
defined. Feces,
nasal
secretions,
saliva, sputum,
sweat, tears,
urine, and
vomitus are not
considered
infectious
unless visibly
bloody.
Exposures that
pose a risk for
transmission
include
percutaneous
injuries,
contact of
mucous
membranes, or
contact of
nonintact skin
with potentially
infected fluids
(22--24).
In studies of
health-care
personnel, the
average risk for
HIV transmission
has been
estimated to be
approximately
0.3% (95%
confidence
interval [CI] =
0.2%--0.5%)
after a
percutaneous
exposure to
HIV-infected
blood and
approximately
0.09% (95% CI =
0.01%--0.5%)
after a mucous
membrane
exposure.
Transmission
risk from
nonintact skin
exposure has not
been quantified
but is estimated
to be less than
that for mucous
membrane
exposure. Risk
following
percutaneous
exposure is
correlated
positively with
exposure to a
larger quantity
of blood, direct
penetration of a
vein or artery,
a deep tissue
injury, or
exposure to
blood from a
source person
with terminal
illness (25),
presumably
related to high
viral load.
Use of PEP
with
antiretroviral
medications,
initiated as
soon as possible
after exposure
and continuing
for 28 days, has
been associated
with a decreased
risk for
infection
following
percutaneous
exposure in
health-care
settings (22).
PEP also is
recommended
following
nonoccupational
sexual and
injection-drug
use--related
exposures (24).
Because of the
potential
toxicities of
antiretroviral
drugs, PEP is
recommended
unequivocally
only for
exposures to
sources known to
be HIV-infected.
The decision to
use PEP
following
unknown-source
exposures is to
be made on a
case-by-case
basis,
considering the
information
available about
the type of
exposure, known
risk
characteristics
of the source,
and prevalence
in the setting
concerned.
In the
majority of
instances
involving
bombings or
other
mass-casualty
events, the
working group
concluded that
the risk for
exposure to
HIV-infected
materials
probably is low
and that
therefore PEP is
not indicated.
On this basis,
PEP is not
routinely
recommended for
treating persons
injured in
mass-casualty
settings in the
United Kingdom (7).
For the same
reason, HIV PEP
should not be
administered
universally in
mass-casualty
settings in the
United States
unless
recommended by
the local public
health
authority. Such
instances might
occur for
mass-casualty
events in
certain specific
settings judged
by public health
authorities to
be associated
with higher risk
for HIV exposure
(e.g., a
research
facility that
contained a
large archive of
HIV-infected
blood
specimens). In
the rare
situation in
which PEP is
recommended, it
should be
initiated as
soon as possible
after exposure,
and specimens
from the exposed
person should be
collected for
baseline HIV
testing.
However, PEP
should not be
delayed for the
results of
testing. If PEP
is used, certain
other laboratory
studies also are
indicated.
Consultation
from health-care
professionals
knowledgeable
about HIV
infection is
ideal, and is
particularly
important for
pediatric
patients and
pregnant women.
All persons for
whom HIV PEP has
been initiated
should be
referred to a
clinician
experienced in
HIV care for
follow up.
Tetanus
Clostridium
tetani, the
causative agent
of tetanus, is
ubiquitous in
the environment
and distributed
worldwide. The
organism is
found in soil
and in the
intestines of
animals and
humans. When
spores of C.
tetani are
introduced into
the anaerobic or
hypoaerobic
conditions found
in wounds or
devitalized
tissue, they
germinate to
vegetative
bacilli that
elaborate toxin
and cause
disease. This
now infrequent
but often fatal
disease has been
associated with
injuries to
otherwise
healthy persons,
particularly
during military
conflicts.
During
1998--2000, the
case-fatality
ratio for
reported tetanus
in the United
States was 18% (26).
Although tetanus
is not
transmitted from
person to
person,
contamination of
wounds with
debris might
increase the
risk for tetanus
among persons
injured in
mass-casualty
settings. Proper
wound care and
debridement play
a critical role
in tetanus
prevention.
Serologic
tests indicate
that immunity to
tetanus toxin is
not acquired
naturally.
However,
protection
against tetanus
is achievable
almost
universally by
use of highly
immunogenic and
safe tetanus
toxoid--containing
vaccines. The
disease now
occurs almost
exclusively
among persons
who were not
vaccinated
adequately or
whose
vaccination
histories are
unknown or
uncertain (27,28).
Universal
primary
vaccination,
with subsequent
maintenance of
adequate
antitoxin levels
by means of
appropriately
timed boosters,
protects persons
among all age
groups.
The age
distribution of
recent cases and
the results of
serosurveys
indicate that
many U.S. adults
are not
protected
against tetanus
(29). The
proportions of
persons lacking
protective
levels of
circulating
antitoxins
against tetanus
increase with
age; at least
40% of persons
aged >60
years might lack
protection. In
the United
States, tetanus
is primarily a
disease of older
adults (27,28).
Children are
much more likely
to have received
age-appropriate
vaccination;
rates for
receipt of 3
doses among
children aged
19--35 months
exceed 96% (28).
During
1992--2000, only
15 cases of
tetanus were
reported in the
United States
among children
aged <15 years.
Parental
philosophic or
religious
objection to
vaccination
accounted for
the absence of
immune
protection for
12 (80%)
affected
children (30).
Foreign-born
immigrants,
especially those
from regions
other than North
America or
Europe, also
might be
relatively
undervaccinated
(29,31).
Available
evidence
indicates that
complete primary
vaccination with
tetanus toxoid
provides
long-lasting
protection.
After routine
childhood
tetanus
vaccination, the
Advisory
Committee on
Immunization
Practices (ACIP)
recommends
routine booster
vaccination with
tetanus
toxoid--containing
vaccines every
10 years. For
clean and minor
wounds, a
booster dose is
recommended if
the patient has
not received a
dose within 10
years. For all
other wounds, a
booster is
appropriate if
the patient has
not received
tetanus toxoid
during the
preceding 5
years.
In the
setting of acute
response to a
mass-casualty
event, failure
to provide a
tetanus
vaccination when
needed could
result in
preventable
illness, whereas
unnecessary
vaccination is
unlikely to
cause harm (26--29,32,33).
A substantial
proportion of
patients in this
setting might be
unable to
provide a
history of
vaccination or
history of
contraindications
to tetanus
toxoid--containing
vaccines, and
the majority of
wounds sustained
will be
considered
tetanus-prone
because they are
likely to be
exposed to dirt
or feces. Thus,
a wounded adult
patient who
cannot confirm
receipt of a
tetanus booster
during the
preceding 5
years should be
vaccinated with
tetanus and
diphtheria
toxoids vaccine
(Td) or tetanus
toxoid, reduced
diphtheria
toxoid, and
acellular
pertussis
vaccine (Tdap);
adults aged >65
years should
receive Td (26).
Similarly, a
child with an
uncertain
vaccination
history should
receive a
tetanus booster
as age-indicated
by the standard
childhood
immunization
table (pediatric
diphtheria and
tetanus toxoids
and acellular
pertussis
vaccine [DTaP]
if aged <7
years, Td if
aged 7--10
years, and Tdap
if aged >11
years) (32,34).
ACIP
recommends that
patients without
a complete
primary tetanus
series who
sustain a
tetanus-prone
wound routinely
receive passive
immunization
with tetanus
immune globulin
(TIG) and
tetanus toxoid (33).
In the setting
of acute
response to a
mass-casualty
event, many
wounded patients
probably will be
unable to
confirm previous
vaccination
histories, and
thus TIG
normally would
be indicated.
However, this
might not be
feasible in a
mass-casualty
setting if
supplies of TIG
are limited. All
decisions to
administer TIG
depend on the
number of
casualties and
the readily
available supply
of TIG. If the
supply of TIG is
adequate,
consideration
might be given
to providing
both tetanus
toxoid and
passive
immunization
with TIG at the
time of
management of
tetanus-prone
wounds. TIG is
indicated if
completion of a
primary
vaccination
series is
uncertain for an
adult or if
prior receipt of
age-appropriate
vaccinations is
uncertain for a
child. If TIG is
in short supply,
it should be
reserved for
patients least
likely to have
received
adequate primary
vaccination. In
general, this
group includes
persons aged
>60 years
and immigrants
from regions
other than North
America or
Europe who might
be less likely
to have adequate
antitetanus
antibodies and
who thus would
derive the most
benefit from TIG
(32).
The TIG
prophylactic
dose that is
recommended
currently for
wounds is 250
units
administered
intramuscularly
(IM) for adult
and pediatric
patients. When
tetanus toxoid
and TIG are
administered
concurrently,
separate
syringes and
separate sites
should be used (35).
In circumstances
in which passive
protection is
clearly
indicated but
TIG is
unavailable,
intravenous
immune globulin
may be
substituted for
TIG.
Postexposure
chemoprophylaxis
with
antimicrobials
against tetanus
is not
recommended.
ACIP
recommends that
adults and
adolescents with
a history of
uncertain or
incomplete
primary
vaccination
complete a
3-dose primary
series for
tetanus,
diphtheria, and
pertussis (26,30--34).
In the setting
of acute
response to a
mass-casualty
event,
completion of
the primary
vaccination
series of any
vaccine provided
initially during
acute response
during follow-up
visits should be
ensured at the
time of
discharge for
inadequately
vaccinated
patients of all
ages. Special
precautions
regarding
management of
pregnant women
in the setting
of emergency
delivery have
been identified
(see Special
Situations).
Recommendations
Risk
Assessment
To determine
appropriate
actions in
response to
evaluation of
casualties of
bombings or
other
mass-casualty
events,
health-care
providers should
- assess
individual
exposure
risk by
categorizing
the patient
into one of
three
exposure
risk
categories (Box
1) that
are numbered
sequentially
from the
highest
(category 1)
to the
lowest
(category 3)
level of
exposure
risk and
assign each
person to
the highest
level risk
category for
which he/she
qualifies,
- identify
the
appropriate
risk
category-
and
pathogen-specific
management
recommendation(s)
(Box
1), and
-
determine
the
appropriate
action to
take (see
Pathogen-Specific
Management
Recommendations)
in response
to
management
recommendations.
When evaluating
management
choices for
casualties of
bombings or
other
mass-casualty
events,
health-care
providers should
assume that
exposure to
blood from other
injured persons
is likely unless
available
information on
the
circumstances of
injury suggests
otherwise. Blast
injuries result
occasionally in
traumatic
implantation of
bone or other
biologic
material that is
alien to the
wounded person.
Testing of such
matter is not
recommended as a
useful adjunct
for clinical
management of
wounded persons.
Public health
authorities can
provide
assistance in
assessing
exposure risk
for affected
groups of
injured persons.
Tetanus risk is
not dependent
upon blood
exposure.
Pathogen-Specific
Management
Recommendations
Hepatitis B
Virus
Unless an
injured person
who is unable to
communicate an
accurate medical
history or for
whom medical
records are not
readily
available is
accompanied by a
person able to
function as a
health-care
proxy,
responders
should assume
the absence of a
reliable
hepatitis B
vaccination
history and no
contraindication
to vaccination
with hepatitis B
vaccine (see
Contraindications
and
Precautions). If
administration
of hepatitis B
vaccine to a
large number of
persons after a
mass-casualty
event is
anticipated to
result in
shortages of
hepatitis B
vaccine
products, or if
such shortages
already exist,
assistance with
vaccine supply
is available
(see Vaccine
Supply).
Recommendation:
Intervene:
- Persons
for whom
neither a
reliable
history of
completed
vaccination
against HBV
nor a known
contraindication
to
vaccination
against HBV
exist should
receive the
first dose
of the HBV
vaccine
series as
soon as
possible
(preferably
within 24
hours) and
not later
than 7 days
after the
event.
- Persons
who receive
or are
identified
as
candidates
for a dose
of hepatitis
B vaccine
while
undergoing
evaluation
or treatment
in immediate
response to
a
mass-casualty
event should
be
discharged
with
referrals
for
follow-up
and written
information
on
predischarge
treatment to
facilitate
the ability
of primary
health-care
providers to
evaluate
and, if
appropriate,
initiate or
complete
age-appropriate
vaccinations
or
vaccination
series (Appendix
1).
Recommendation:
No action:
- No
action is
necessary to
prevent HBV
infection.
Hepatitis C
Virus
Recommendation:
Consider
testing:
- Testing
should be
considered
when an
HCV-infected
source is
known or
thought to
be likely on
the basis of
the setting
in which the
injury
occurred or
exposure to
blood or
biologic
material
from a
bomber or
multiple
other
injured
persons is
suspected.
- Public
health
authorities
can provide
assistance
in assessing
exposures
and
therefore
treatment
for affected
groups of
injured
persons. A
decision to
perform
testing of
specific
persons
might be
made on the
basis of the
judgment of
the treating
physician
and the
preferences
of the
individual
patient;
testing
during a
follow-up
referral
might be a
more
feasible
logistical
option in
the setting
of response
to a
mass-casualty
event.
If a decision is
made to perform
testing:
- baseline
testing for
anti-HCV and
alanine
aminotransferase
(ALT) should
be performed
within 7--14
days of the
exposure;
-
follow-up
testing for
anti-HCV and
ALT should
be performed
4--6 months
after
exposure to
assess
seroconversion,
preferably
arranged as
part of
discharge
planning;
- HCV RNA
testing
should be
performed at
4--6 weeks
if an
earlier
diagnosis of
HCV
infection is
desired; and
- positive
anti-HCV
with low
signal-to-cutoff
value should
be confirmed
using a more
specific
supplemental
assay before
communicating
the results
to the
patient; and
- persons
who are
tested or
are
identified
as a
candidate
for testing
regarding
exposure to
HCV while
undergoing
evaluation
or treatment
in immediate
response to
a
mass-casualty
event should
be
discharged
with a
referral for
follow-up
and written
information
on
pre-discharge
treatment (Appendix
1).
Recommendation:
Generally no
action:
- Exposure
of mucous
membranes to
blood from a
source with
unknown HCV
status
generally
poses a
minor risk
for
infection
and does not
require
further
action.
- However,
in settings
in which
exposure to
an
HCV-infected
source is
known or
thought to
be highly
likely,
testing for
early
identification
of HCV
infection
following
mucous
membrane
exposure may
be
considered.
The decision
to perform
testing
should be
made on the
basis of the
judgment of
the treating
physician
and the
preference
of the
individual
patient.
Recommendation:
No action
- No
action is
necessary to
prevent HCV
infection.
Human
Immunodeficiency
Virus
Recommendation:
Generally no
action:
- In
general, HIV
PEP is not
warranted.
HIV PEP
might be
considered
only in
settings in
which
exposure to
an
HIV-infected
source is
known or
thought to
be highly
likely
(e.g., a
blast injury
incident
that
occurred in
a research
facility
that
contained a
large
archive of
HIV infected
blood
specimens).
- HIV PEP
should not
be
administered
universally
in response
to
mass-casualty
events
unless
recommended
by the local
public
health
authority.
- In the
rare event
that HIV PEP
is
considered,
it should be
initiated as
soon as
possible
after
exposure.
The patient
should be
counseled
about the
availability
of PEP and
informed of
the
potential
benefits and
risks and
the need for
prompt
initiation
to maximize
potential
effectiveness.
If PEP is
thought to
be indicated
on the basis
of exposure
risk,
administration
should not
be delayed
for HIV test
results.
Specific
guidance on
how to
administer
HIV PEP in
unusual
circumstances
when it is
warranted is
available
(see Special
Situations).
- Persons
who receive
or are
identified
as
candidates
for HIV PEP
while
undergoing
evaluation
or treatment
in immediate
response to
a
mass-casualty
event should
be
discharged
with
referrals
for urgent
follow-up.
Written
information
on
predischarge
treatment
should be
provided to
facilitate a
primary
health-care
provider's
ability to
evaluate
and, if
appropriate,
complete
age-appropriate
vaccinations
or
vaccination
series (Appendix
1).
- In all
health-care
settings,
opt-out
screening
for HIV
(performing
HIV
screening
after
notifying
the patient
that the
test will be
performed,
with assent
inferred
unless the
patient
declines or
defers
testing) is
recommended
for all
patients
aged 13--64
years. In
the setting
of response
to a
mass-casualty
event,
testing
during a
follow-up
referral
might be a
more
feasible
logistic
option
unless a
decision to
administer
PEP has been
made (35).
Recommendation:
No action:
- No
action is
necessary to
prevent HIV
infection.
Tetanus
All persons
who sustain
tetanus-prone
injuries in
mass-casualty
settings should
be evaluated for
the need for
tetanus
prophylaxis.
Tetanus-prone
injuries include
but are not
limited to
puncture and
other
penetrating
wounds with the
potential to
result in an
anaerobic
environment
(wounds
resulting from
projectiles or
by crushing) and
wounds,
avulsions,
burns, or other
nonintact skin
that might be
contaminated
with feces, soil
or saliva.
All persons
who are not
accompanied by
either medical
records or a
health-care
proxy and whose
ability to
communicate an
accurate medical
history is
uncertain for
any reason
should be deemed
to lack a
reliable tetanus
toxoid
vaccination
history and to
have no
contraindication
to vaccination
with tetanus
toxoid (see
Contraindications
and
Precautions). If
compliance with
recommendations
is anticipated
to result in a
shortage of
tetanus toxoid
products or TIG,
assistance with
product supplies
is available
(see Vaccine
Supply).
Recommendation:
Intervene:
-
Appropriate
wound care
and
debridement
are critical
to tetanus
prevention.
-
Age-appropriate
vaccines
should be
used if
possible.
However, in
a
mass-casualty
setting,
this might
not be
possible,
and any
tetanus
vaccine
formulation
might be
used,
because the
tetanus
toxoid
content is
adequate for
tetanus
prophylaxis
in any age
group. In
this
setting, the
benefit of
supplying
tetanus
prophylaxis
outweighs
the
potential
for adverse
reactions
from
formulations
from a
different
age
indication.
- Adult
patients who
cannot
readily
confirm
receipt of a
tetanus
booster
during the
preceding 5
years and
who do not
have known
contraindication
to tetanus
vaccination
should be
vaccinated
with Tdap
(or with Td
if Tdap is
unavailable)
or with Td
if aged >65
years.
-
Pediatric
patients
with
uncertain
vaccination
history and
with no
known
contraindication
to tetanus
vaccination
should
receive a
tetanus
booster
according to
the
following
schedule:
--- DTaP if
aged <7
years
--- Td if
aged 7--10
years
--- Tdap (or
Td if Tdap
is
unavailable)
if aged >11
years.
- In a
mass-casualty
situation,
unusually
high demand
might result
in shortages
of
age-specific
vaccine
formulations,
and logistic
considerations
might make
differentiating
patients by
age category
prohibitive.
If supplies
of DTaP are
inadequate,
heath-care
providers
might
consider
substituting
Tdap or Td
for DTaP
because the
amount of
tetanus
toxoid in
all
formulations
is adequate
to induce an
immune
response in
a child.
Similarly,
if supplies
of Td are
inadequate,
health-care
providers
might
consider
substituting
Tdap for Td
for persons
aged >65
years.
Pediatric
DTaP
generally is
not
indicated in
persons aged
>7
years; the
increased
diphtheria
toxoid
content is
associated
with higher
rates of
local
adverse
reactions in
older
persons (26,32).
However, in
a
mass-casualty
setting,
other
options
might not
exist.
- TIG
might be
indicated if
completion
of a primary
vaccination
series is
uncertain
for an
adult, or
prior
receipt of
age-appropriate
vaccinations
is uncertain
for a child.
--- If TIG
is in short
supply, use
of TIG
should be
reserved
first for
persons aged
>60
years and
for
immigrants
from regions
other than
North
America or
Europe. All
decisions to
administer
TIG depend
on the
number of
casualties
and the
readily
available
supply of
TIG.
--- The
recommended
prophylactic
dose of TIG
is 250 units
IM for adult
and
pediatric
patients.
When tetanus
toxoid and
TIG are
administered
concurrently,
separate
syringes and
separate
sites should
be used (34).
- Persons
who receive
or are
identified
as
candidates
for tetanus
toxoid--containing
products or
TIG while
undergoing
evaluation
or treatment
in immediate
response to
a
mass-casualty
event should
be
discharged
with
referrals
for
follow-up if
possible.
Written
information
on
predischarge
treatment
should be
provided to
facilitate
the ability
of primary
health-care
providers to
evaluate
and, if
appropriate,
complete
age-appropriate
vaccinations
or
vaccination
series (Appendix
1).
Recommendation:
No action:
- No
action is
necessary to
prevent
tetanus.
Exposure to
blood or
other bodily
fluids
generally is
not
considered a
risk factor
for tetanus.
- However,
responders
or persons
engaged in
debris clean
up and
construction
are
candidates
for
prophylaxis
even if they
do not
sustain any
wounds. When
feasible, as
a routine
public
health
measure,
tetanus
toxoid
vaccination
with Tdap or
Td should be
offered to
all persons
whose last
tetanus
toxoid--containing
vaccine was
received
>10
years
previously
and who
either are
responders
or are
engaged in
either
debris
clean-up or
construction
and who thus
might be
expected to
encounter
further risk
for exposure
(36--39).
Vaccine and
Antitoxin Supply
Adherence to
these
recommendations
might increase
the acute demand
for tetanus
toxoid--containing
vaccine, TIG,
and hepatitis B
vaccine beyond
the available
local supply. In
that event,
local
authorities
might have to
rely on local
and state health
departments,
mutual aid
agreements, or
commercial
vendors to
supplement the
supply of needed
biologic or
pharmaceutical
products. If a
local
authority's
capacity to
respond to an
emergency is
exceeded and
other local or
regional
resources are
inadequate,
local and state
public health
jurisdictions
can, through
their
established
communication
channels for
health
emergencies,
work with CDC
and others as
appropriate to
assist with
product
shortages.
CDC's
Strategic
National
Stockpile (SNS)
maintains bulk
quantities of
pharmaceutical
and
nonpharmaceutical
medical supplies
for use in a
national
emergency.
Tetanus toxoid,
tetanus immune
globulin, and
hepatitis B
vaccine are not
included in the
stockpile
formulary.
However, SNS has
purchasing
agreements for
acquiring
medical
materials in
large
quantities,
subject to
commercial
availability.
CDC maintains
stockpiles of
pediatric
vaccine products
purchased by the
Vaccines for
Children Program
that might be
used to assist
state,
territorial, and
tribal health
departments in
meeting emergent
local demands
for vaccines.
CDC also can
work with
manufacturers
and with state
and local health
authorities to
assist with
supply of
vaccines that
are not
available in
either the SNS
or other CDC
vaccine
stockpiles.
Counseling
Hepatitis
B and C Viruses
Persons
undergoing
postexposure
management for
possible
exposure to HBV-
or HCV-infected
blood do not
need to take any
special
precautions to
prevent
secondary
transmission
during the
follow-up period
(12,17).
The exposed
person does not
need to modify
sexual practices
or refrain from
becoming
pregnant. An
exposed nursing
mother might
continue to
breastfeed.
However, exposed
persons should
refrain from
donating blood,
plasma, organs,
tissue, or semen
until follow-up
testing by the
health-care
provider has
excluded
seroconversion (12,17).
Human
Immunodeficiency
Virus
Persons known to
be exposed to
HIV should
refrain from
blood, plasma,
organ, tissue,
or semen
donation until
follow-up
testing by the
health-care
provider has
excluded
seroconversion.
In addition,
measures to
prevent sexual
transmission
(e.g.,
abstinence or
use of condoms)
should be taken,
and
breastfeeding
should be
avoided until
HIV infection
has been ruled
out (22).
Special
Situations
When HIV
PEP is Initiated
HIV PEP should
be considered
only under
exceptional
circumstances.
In the rare
event that HIV
PEP is
considered, it
should be
initiated as
soon as possible
after exposure.
The patient
should be
counseled about
the availability
of PEP and
informed about
the potential
benefits and
risks and the
need for prompt
initiation to
maximize
potential
effectiveness.
If PEP is
thought to be
indicated on the
basis of
exposure risk,
administration
should not be
delayed for HIV
test results.
In the rare
event that HIV
PEP is
administered,
specimens should
be collected for
baseline HIV
testing on all
patients
provided with
PEP using a
blood or oral
fluid rapid test
if available;
otherwise,
conventional
testing should
be used. Testing
should be
discussed with
the patient if
the patient's
medical
condition
permits.
Procedures for
testing should
be in accordance
with applicable
state and local
laws. PEP can be
initiated and
test results
reviewed at
follow-up. If
the HIV test
result is
positive, PEP
can be
discontinued and
the patient
referred to a
clinician
experienced with
HIV care for
treatment.
If PEP is
administered,
the health-care
provider also
should obtain
baseline
complete blood
count, renal
function,
hepatic function
tests, and, in
women, a
pregnancy test.
Because
efavireniz might
be teratogenic,
it should not be
administered
until pregnancy
test results are
available (12,22).
Otherwise, test
results need not
be available
before PEP
initiation but
should be
reviewed in
follow-up.
Selection of
antiretroviral
regimens should
aim for
simplicity and
tolerability.
Because of the
complexity of
selection of HIV
PEP regimens,
consultation
with persons
having expertise
in
antiretroviral
therapy and HIV
transmission is
strongly
recommended.
Resources for
consultation are
available from
the following
sources:
- local
infectious
diseases,
hospital
epidemiology,
or
occupational
health
consultants;
- local,
state, or
federal
public
health
authorities;
- PEPline
at
http://www.nccc.ucsf.edu/Hotlines/PEPline.html,
telephone
888-448-4911;
- HIV/AIDS
Treatment
Information
Service at
http://aidsinfo.nih.gov;
and
-
previously
published
guidance
(see
Information
Sources).
Nevirapine
should not be
included in HIV
PEP regimens
because of
potential severe
hepatic and
cutaneous
toxicity.
Efavirenz should
not be used if
pregnancy is
known or
suspected
because of
potential
teratogenicity (12,22).
PEP should be
started as soon
after exposure
as possible and
continue for 4
weeks. For
ambulatory
patients, a
starter pack of
5--7 days of
medication
should be
provided, if
possible.
Alternatively,
for hospitalized
patients, the
first dose
should be taken
in the emergency
department, and
follow-up orders
should be
written for
completion of
the course in
the hospital.
Patients on
PEP should be
reassessed for
adherence,
toxicity, and
for follow-up of
HIV testing (if
rapid testing
was not
available at
baseline) within
72 hours by an
infectious
disease
consultant.
Patients
continuing on
PEP should have
follow-up
laboratory
evaluation as
recommended
previously (22--24),
including a
complete blood
count and renal
and hepatic
function tests
at baseline and
at 2 weeks
postexposure,
and HIV testing
at baseline, 6
weeks, 3 months,
and 6 months
postexposure.
Persons begun
on HIV PEP
should be
discharged with
written
instructions and
a referral to
ensure follow-up
care with a
clinician
experienced with
HIV care and
information on
the
age-appropriate
dose and
schedule (Appendix
1).
Simultaneous
Administration
When tetanus
toxoid and TIG
are administered
concurrently,
separate
syringes and
separate
anatomic sites
should be used (40).
Hepatitis B
vaccine and
tetanus
toxoid--containing
vaccines might
be administered
at the same time
using separate
syringes and
separate sites (36).
Treatment
with an
antimicrobial
agent generally
is not a
contraindication
to vaccination (40).
Antimicrobial
agents have no
effect on the
responses to
vaccines against
tetanus or
hepatitis B.
Administration
of Blood
Products
The
administration
of hepatitis B
vaccine or
tetanus
toxoid--containing
products does
not need to be
deferred in
persons who have
received a blood
transfusion or
other blood
products.
Pregnancy
Pregnancy is not
a
contraindication
to vaccination
against
hepatitis B.
Limited data
suggest that a
developing fetus
is not at risk
for adverse
events when
hepatitis B
vaccine is
administered to
a pregnant
woman. Available
vaccines contain
noninfectious
HBsAg and should
cause no risk
for infection to
the fetus (11).
Pregnancy is
not a
contraindication
for HIV PEP.
However, use of
efavirenz should
be avoided when
pregnancy is
known or
suspected (11,22).
Pregnant
adolescents and
adults who
received the
most recent
tetanus
toxoid--containing
vaccine >5
years previously
generally should
receive Td in
preference to
Tdap when
possible (41).
Responders and
Other Personnel
Responders and
persons engaged
in debris
removal or
construction
often are at
risk for
incurring wounds
throughout the
duration of
response and
clean up work.
As a routine
public health
measure,
health-care
providers should
offer tetanus
toxoid
vaccination to
all response
workers who do
not have a
reliable history
of receipt of a
tetanus
toxoid--containing
vaccine during
the preceding 10
years,
regardless of
whether the
health-care
visit was for a
wound (38,39).
Such persons
might encounter
potential
exposure
situations
throughout the
duration of
their work in
response to a
mass-casualty
situation.
Health-care
personnel,
emergency
response, public
safety and other
workers (e.g.,
construction
workers and
equipment
operators) who
are injured and
exposed to blood
while providing
assistance after
a mass-casualty
event should be
managed
according to
existing
guidelines and
standards for
the management
of occupational
exposures (10,22,42).
Health-care
personnel and
first responders
whose activities
involve contact
with blood or
other body
fluids should
have been
previously
vaccinated
against HBV and
tetanus (12,22).
Contraindications
and Precautions
Hepatitis
B Vaccine
Hepatitis B
vaccination is
contraindicated
for persons with
a history of
anaphylactic
allergy to yeast
or any vaccine
component (11).
On the basis of
CDC's Vaccine
Study Datalink
data, the
estimated
incidence of
anaphylaxis
among children
and adolescents
who received
hepatitis B
vaccine is 1
case per 1.1
million vaccine
doses
distributed (95%
CI = 0.1--3.9) (11).
Persons with a
history of
serious adverse
events (e.g.,
anaphylaxis)
after receipt of
hepatitis B
vaccine should
not receive
additional
doses.
Vaccination is
not
contraindicated
in persons with
a history of
multiple
sclerosis,
Guillain-Barré
syndrome,
autoimmune
disease (e.g.,
systemic lupus
erythematosis or
rheumatoid
arthritis), or
other chronic
diseases (11).
Antiretroviral
Therapy
Nevirapine
should not be
included in HIV
PEP regimens
because of
potential severe
hepatic and
cutaneous
toxicity.
Efavirenz should
not be used if
pregnancy is
known or
suspected
because of
potential
teratogenicity (12,22).
Preparations
Containing
Tetanus Toxoid
The only
contraindication
to preparations
containing
tetanus toxoid
(TT, Td, or
Tdap) is a
history of a
neurologic or
severe allergic
reaction
following a
previous dose.
Local side
effects alone do
not preclude
continued use (26,30,31).
If a person has
a wound that is
neither clean
nor minor and
for which
tetanus
prophylaxis is
indicated, but
also a
contraindication
to receipt of
tetanus
toxoid--containing
preparations,
only passive
immunization
using human TIG
should be
administered.
Reporting
Adverse Events
Vaccine
Adverse Events
Reporting System
Any clinically
significant
adverse events
that occur after
administration
of any vaccine
should be
reported to the
Vaccine Adverse
Events Reporting
System (VAERS)
even if causal
relation to
vaccination is
uncertain. The
National
Childhood
Vaccine Injury
Act requires
health-care
providers to
report to VAERS
any event listed
by the vaccine
manufacturers as
a
contraindication
to subsequent
doses of the
vaccine or any
event listed in
the Reportable
Events Table
(available at
http://vaers.hhs.gov/reportable.htm)
that occurs
within the
specified period
after
vaccination.
VAERS reporting
forms and
information can
be requested 24
hours a day at
telephone
800-822-7967 or
by accessing
VAERS at
http://vaers.hhs.gov.
Web-based
reporting also
is available,
and providers
are encouraged
to report
adverse events
electronically
at
http://secure.vaers.org/VaersDataEntryintro.htm.
Reporting
Adverse Events
Associated With
Antiretroviral
Drugs and TIG
Unusual or
severe
toxicities
believed to be
associated with
use of
antiretroviral
agents or TIG
should be
reported to
FDA's MEDWATCH
program (http://www.fda.gov/medwatch)
at MedWatch,
HF-2, Food and
Drug
Administration,
5600 Fishers
Lane, Rockville,
MD 20857;
telephone
800-332-1088.
National
Vaccine Injury
Compensation
Program
The National
Vaccine Injury
Compensation
Program (NVICP)
was established
by the National
Childhood
Vaccine Injury
Act and became
operational on
October 1, 1988.
Intended as an
alternative to
civil litigation
under the
traditional tort
system (in that
negligence need
not be proven),
NVICP is a
no-fault system
in which persons
thought to have
suffered an
injury or death
as a result of
administration
of a covered
vaccine may seek
compensation.
Claims may be
filed on behalf
of infants,
children and
adolescents, or
by adults
receiving
VICP-covered
vaccines. Other
legal
requirements
(e.g., the
statute of
limitations for
filing an injury
or death claim)
must be
satisfied to
pursue
compensation.
Claims arising
from covered
vaccines must be
adjudicated
through the
program before
civil litigation
can be pursued.
The program
relies on a
Reportable
Events Table
listing the
vaccines covered
by the program
and the
injuries,
disabilities,
illnesses, and
conditions
(including
death) for which
compensation
might be
awarded.
Additional
information
about NVICP is
available at
http://www.hrsa.gov/vaccinecompensation
or from the
National Vaccine
Injury
Compensation
Program, Health
Resources and
Services
Administration,
Parklawn
Building, Room
11C-26, 5600
Fishers Lane,
Rockville, MD
20857; telephone
800-338-2382.
Information
Sources
Recommendations
for immediate
prophylactic
interventions
have been
summarized (Table
2).
Recommendations
for issues that
might arise in
association with
immediate
prophylactic
intervention
also have been
summarized (Table
3).
In addition
to the guidance
provided in
these
recommendations,
information on
specific
vaccines or
other
prophylactic
interventions
also is
available (Box
2). ACIP
recommendations
regarding
vaccine use are
published by
MMWR.
Electronic
subscriptions
are available
free of charge
at
http://www.cdc.gov/subscribe.html.
Printed
subscriptions
are available at
Superintendent
of Documents,
U.S. Government
Printing Office,
Washington, D.C.
20402-9235,
telephone
202-512-1800.
Acknowledgments
Contributors
to this report
included the
National
Association of
County and City
Health
Officials, the
Council of State
and Territorial
Epidemiologists,
the Terrorism
Injuries:
Information
Dissemination
and Exchange
(TIIDE)
partnership, and
the following
persons: Italo
Subbarao, DO,
Center for
Public Health
Preparedness and
Disaster
Response,
American Medical
Association;
Richard Sattin,
MD, Division of
Injury Response,
National Center
for Injury
Prevention and
Control; Richard
McCluskey, MD,
Coordinating
Office for
Terrorism
Preparedness and
Emergency
Response; Jeanne
Santoli, MD,
Immunization
Services
Division,
National Center
for
Immunizations
and Respiratory
Diseases;
William
Atkinson, MD,
Immunization
Services
Division,
National Center
for
Immunizations
and Respiratory
Diseases, CDC.
References
-
Federal
Bureau of
Investigation.
Terrorism
2002--2005.
Washington,
DC: US
Department
of Justice,
Federal
Bureau of
Investigation;
2006.
Available at
http://www.fbi.gov/publications.terror/terrorism2002_2005.htm.
-
Siegel-Itzkovich
J. Israeli
minister
orders
hepatitis B
vaccine for
survivors of
suicide bomb
attacks. BMJ
2001;323:417.
-
Braverman I,
Wexler D,
Oren M. A
novel mode
of infection
with
hepatitis B:
penetrating
bone
fragments
due to the
explosion of
a suicide
bomber. Isr
Med Assoc J
2002;4:528--9.
- Wong J
M-l, Marsh
D, Abu-Sitta
G, et al.
Biological
foreign body
implantation
in victims
of the
London July
7th suicide
bombings. J
Trauma
2006;60:402--4.
- Israel
Ministry of
Health.
Hepatitis B
vaccination
protocol in
survivors of
mass
casualty
bombings.
Israel
Ministry of
Health
Medical
Guidelines
2001(35)/1/13(Communique
57/2001).
- Israel
Ministry of
Health.
Hepatitis B
vaccination
protocol in
survivors of
mass
casualty
bombings--revision.
Israel
Ministry of
Health
Medical
Guidelines
2001(35)/2/13(Communique
72/2001).
- Health
Protection
Agency. Post
exposure
prophylaxis
against
hepatitis B
for bomb
victims and
immediate
care
providers.
Consideration
of other
blood borne
viruses
(hepatitis C
and HIV).
London,
United
Kingdom:
Health
Protection
Agency;
2005.
- Jagger
J, De Carli
G, Perry J,
Puro V,
Ippolito G.
Occupational
exposure to
bloodborne
pathogens:
epidemiology
and
prevention.
In: Wenzel
R, ed.
Prevention
and control
of
nosocomial
infections.
4th ed.
Baltimore,
MD:
Lippincott,
Williams and
Wilkins;
2003.
- Alter
HJ, Stramer
SL, Dodd RY.
Emerging
infectious
diseases
that
threaten the
blood
supply. 1.
Semin
Hematol
2007;44:32--41.
-
CDC.
Prevention
of hepatitis
A through
active or
passive
immunization:
recommendations
of the
Advisory
Committee on
Immunization
Practices
(ACIP). MMWR
2006;55(No.
RR-7).
-
CDC. A
comprehensive
immunization
strategy to
eliminate
transmission
of hepatitis
B virus
infection in
the United
States:
recommendations
of the
Advisory
Committee on
Immunization
Practices
(ACIP). Part
II:
Immunization
of adults.
MMWR
2006;55(No.
RR-16).
-
CDC. Updated
U. S. Public
Health
Service
guidelines
for the
management
of
occupational
exposures to
HBV, HCV,
and HIV and
recommendations
for
postexposure
prophylaxis.
MMWR
2001;50(No.
RR-11).
-
CDC.
National,
state, and
local area
vaccination
coverage
among
children
aged 19--35
months---United
States,
2006. MMWR
2007:56:880--5.
-
CDC.
National
vaccination
coverage
among
adolescents
aged 13--17
years---United
States,
2006. MMWR
2007;56:885--8.
-
CDC.
Hepatitis B
vaccination
among
adults---United
States,
2004. MMWR
2006;55:509--11.
-
Armstrong
GL, Wasley
A, Simard
EP,
McQuillan
GM, Kuhnert
WL, Alter
MJ. The
prevalence
of hepatitis
C virus
infection in
the United
States, 1999
through
2002. Ann
Intern Med
2006;144:705--14.
-
CDC.
Recommendations
for
prevention
and control
of hepatitis
C virus
(HCV)
infection
and
HCV-related
chronic
disease.
MMWR
1998;47(No.
RR-19).
- Beltrami
EM, Kozak A,
Williams IT,
et al.
Transmission
of HIV and
hepatitis C
virus from a
nursing home
patient to a
health care
worker. Am J
Infect
Control
2003;31:168--75.
- Kamili
S,
Krawczynski
K,
McCaustland
K, Li X,
Alter MJ.
Infectivity
of hepatitis
C virus in
plasma after
drying and
storing at
room
temperature.
Infect
Control Hosp
Epidemiol
2007;28:519--24.
- Patel
PR, Larson
AK, Castel
AD, et al.
Hepatitis C
virus
infections
from a
contaminated
radiopharmaceutical
used in
myocardial
perfusion
studies.
JAMA
2006;296:2005--11.
- CDC.
HIV/AIDS
surveillance
report,
cases of HIV
infection
and AIDS in
the United
States and
dependent
areas, 2005.
Vol 17.
Revised June
2007.
Atlanta, GA:
US
Department
of Health
and Human
Services,
Public
Health
Service,
CDC; 2007.
-
CDC. Updated
U.S. Public
Health
Service
guidelines
for the
management
of
occupational
exposures to
HIV and
recommendations
for
postexposure
prophylaxis.
MMWR
2005;54(No.
RR-9).
-
CDC. Notice
to readers:
updated
information
regarding
antiretroviral
agents used
as HIV
postexposure
prophylaxis
for
occupational
HIV
exposures.
MMWR
2007;56;1291--2.
-
CDC.
Antiretroviral
postexposure
prophylaxis
after
sexual,
injection-drug
use, or
other
nonoccupational
exposure to
HIV in the
United
States. MMWR
2005;54(No.
RR-2).
- Bell DM.
Occupational
risk of
human
immunodeficiency
virus
infection in
health-care
workers: an
overview. Am
J Med
1997;102:9--15.
-
CDC.
Preventing
tetanus,
diphtheria
and
pertussis
among
adults: use
of tetanus
toxoid,
reduced
diphtheria
toxoid and
acellular
pertussis
vaccines:
recommendations
of the
Advisory
Committee on
Immunization
Practices
(ACIP) and
Recommendation
of ACIP,
supported by
the
Heatlhcare
Infection
Control
Practices
Advisory
Committee
(HICPAC),
for use of
Tdap among
health-care
personnel.
MMWR
2006;55(No.
RR-17).
-
Pascual FB,
McGinley E,
Zanardi L,
et al.
Tetanus
surveillance---United
States,
1998--2000.
In: CDC
Surveillance
Summaries,
June 20,
2003. MMWR
2003;52(No.
SS-3).
-
Srivastava
P, Brown K,
Chen J,
Kretsinger
K, Roper M.
Trends in
tetanus
epidemiology
in the
United
States,
1972--2001
[Presentation].
Presented at
the 39th
National
Immunization
Conference,
Washington,
DC; March
21--24,
2005.
Available at
http://cdc.confex.com/cdc/nic2005/techprogram/paper_7813.htm.
-
McQuillan
GM,
Kruszon-Moran
D, Deforest
A, et al.
Serologic
immunity to
diphtheria
and tetanus
in the
United
States. Ann
Intern Med
2002;136:660--6.
- Fair E,
Murphy T,
Golaz A,
Wharton M.
Philosophic
objection to
vaccination
as a risk
factor for
tetanus
among
children
younger than
15 years.
Pediatrics
2002;109:E2.
- Talan
DA,
Abrahamian
FM, Moran
GJ, et al.
Tetanus
immunity and
physician
compliance
with tetanus
prophylaxis
practices
among
emergency
department
patients
presenting
with wounds.
Ann Emerg
Med
2004;43:305--11.
-
CDC.
Preventing
tetanus,
diphtheria,
and
pertussis
among
adolescents:
use of
tetanus
toxoid,
reduced
diphtheria
toxoid and
acellular
pertussis
vaccines:
recommendations
of the
Advisory
Committee on
Immunization
Practices
(ACIP). MMWR
2006;55(No.
RR-3).
-
CDC.
Diphtheria,
tetanus, and
pertussis:
recommendations
for vaccine
use and
other
preventive
measures:
recommendations
of the
Immunization
Practices
Advisory
Committee
(ACIP). MMWR
1991:40(No.
RR-10).
- CDC.
Childhood,
adolescent
and adult
immunization
schedules.
Atlanta, GA:
US
Department
of Health
and Human
Services,
CDC; 2007.
Available at
http://www.cdc.gov/vaccines/recs/schedules/default.htm.
-
CDC. Revised
recommendations
for HIV
testing of
adults,
adolescents,
and pregnant
women in
health-care
settings.
MMWR
2006;55(No.
RR-14).
- CDC.
Interim
immunization
recommendations
for disaster
responders:
Hurricane
Katrina.
Atlanta, GA:
US
Department
of Health
and Human
Services,
CDC; 2005.
Available at
http://www.bt.cdc.gov/disasters/disease/responderimmun.asp.
- CDC.
Emergency
wound care
after a
natural
disaster.
Atlanta, GA:
US
Department
of Health
and Human
Services,
CDC; 2005.
Available at
http://www.bt.cdc.gov/disasters/woundcare.asp.
- CDC.
Update:
NIOSH warns
of hazards
of flood
cleanup
work.
Atlanta, GA:
US
Department
of Health
and Human
Services,
CDC; 1997.
NIOSH
publication
no. 94-123.
Available at
http://www.cdc.gov/niosh/flood.html.
-
Occupational
Safety and
Health
Administration.
Fact sheets
on natural
disaster
recovery:
flood
cleanup.
Washington,
DC: US
Department
of Labor,
Occupational
Safety and
Health
Administration;
2008.
Available at
http://www.osha.gov/OshDoc/floodCleanup.html.
-
CDC. General
recommendations
on
immunization.
recommendations
of the
Advisory
Committee on
Immunization
Practices
(ACIP). MMWR
2006;55(No.
RR-15).
-
CDC.
Prevention
of
pertussis,
tetanus, and
diphtheria
among
pregnant and
postpartum
women and
their
infants:
recommendations
of the
Advisory
Committee on
Immunization
Practices
(ACIP). MMWR
2008;57(No.
RR-4).
-
Bloodborne
Pathogens
Standard, 29
C.F.R. Sect.
1910.1030
[56 FR
64004, Dec.
06, 1991, as
amended at
57 FR 12717,
April 13,
1992; 57 FR
29206, July
1, 1992; 61
FR 5507,
Feb. 13,
1996; 66 FR
5325 Jan.,
18, 2001; 71
FR 16672 and
16673, April
3, 2006].
Table 1
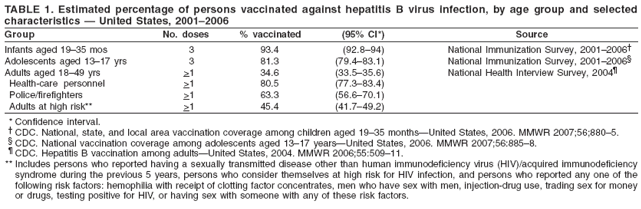
Return to top.
Box 1
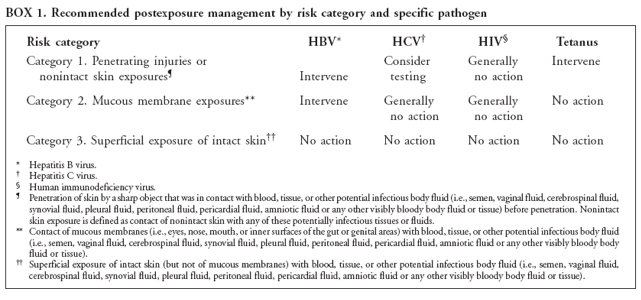
Return to top.
Table 2
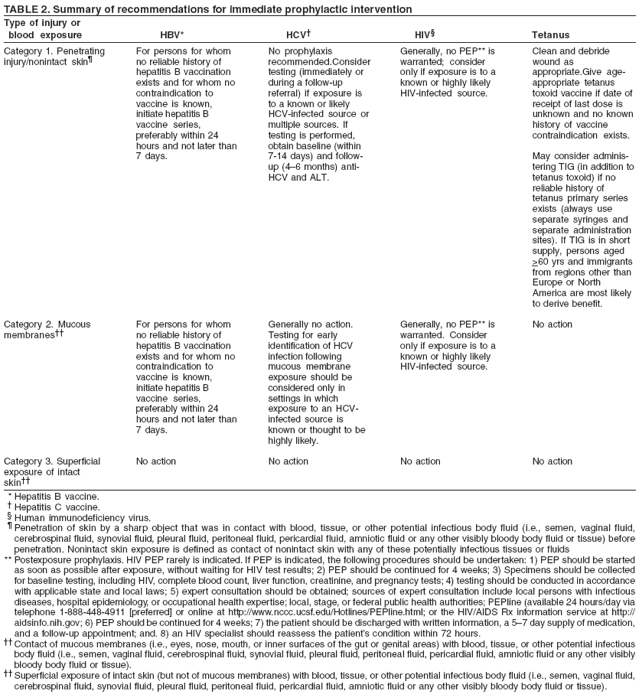
Return to top.
Box 2
Return to top.
Table 3
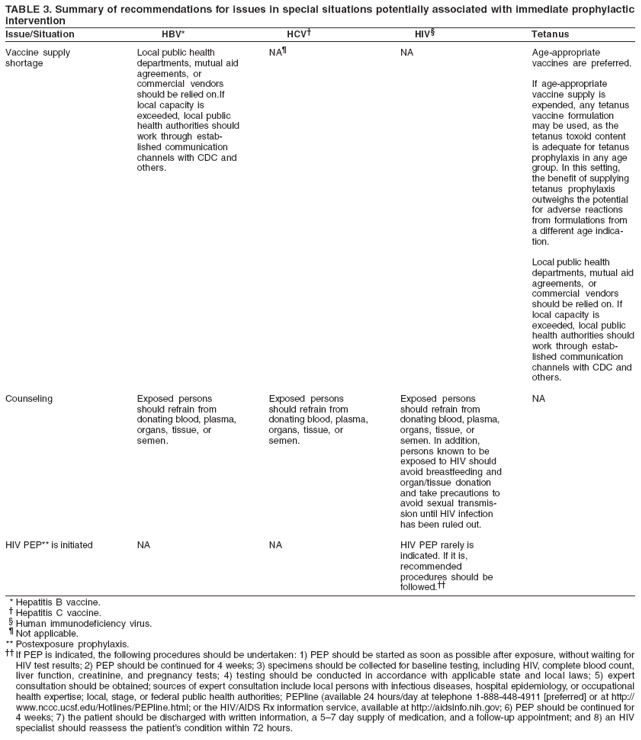
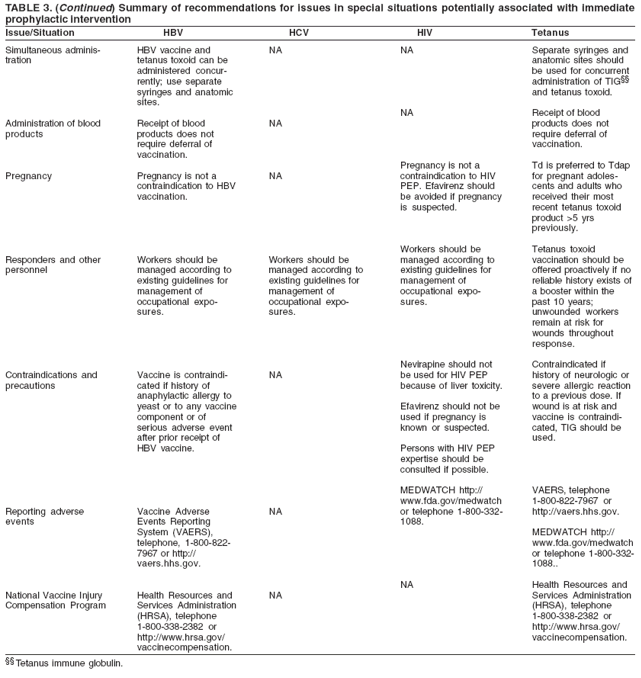
Return to top.
Use of
trade
names
and
commercial
sources
is for
identification
only and
does not
imply
endorsement
by the
U.S.
Department
of
Health
and
Human
Services.
References
to
non-CDC
sites on
the
Internet
are
provided
as a
service
to
MMWR
readers
and do
not
constitute
or imply
endorsement
of these
organizations
or their
programs
by CDC
or the
U.S.
Department
of
Health
and
Human
Services.
CDC is
not
responsible
for the
content
of pages
found at
these
sites.
URL
addresses
listed
in
MMWR
were
current
as of
the date
of
publication.
|
All
MMWR HTML
versions of
articles are
electronic
conversions from
typeset
documents. This
conversion might
result in
character
translation or
format errors in
the HTML
version. Users
are referred to
the electronic
PDF version
(http://www.cdc.gov/mmwr)
and/or the
original MMWR
paper copy for
printable
versions of
official text,
figures, and
tables. An
original paper
copy of this
issue can be
obtained from
the
Superintendent
of Documents,
U.S. Government
Printing Office
(GPO),
Washington, DC
20402-9371;
telephone: (202)
512-1800.
Contact GPO for
current prices.
**Questions or
messages
regarding errors
in formatting
should be
addressed to
mmwrq@cdc.gov.
Date last
reviewed:
7/9/2008



Prepared by
Louisa E.
Chapman, MD1
Ernest E.
Sullivent, MD2
Lisa A.
Grohskopf, MD3
Elise M.
Beltrami, MD4
Joseph F. Perz,
DrPH5
Katrina
Kretsinger, MD6
Adelisa L.
Panlilio, MD4
Nicola D.
Thompson, PhD5
Richard L.
Ehrenberg, MD7
Kathleen F.
Gensheimer, MD8,9
Jeffrey S.
Duchin, MD10,11
Peter H. Kilmarx,
MD3
Richard C. Hunt,
MD2
1Immunization
Services
Division,
National Center
for
Immunizations
and Respiratory
Diseases,
2Division
of Injury
Response,
National Center
for Injury
Prevention and
Control
3Division
of HIV/AIDS
Prevention,
National Center
for HIV/AIDS,
Viral Hepatitis,
STD, and TB
Prevention
4Division
of Healthcare
Quality
Promotion,
National Center
for
Preparedness,
Detection, and
Control of
Infectious
Diseases
5Division
of Viral
Hepatitis,
National Center
for HIV/AIDS,
Viral Hepatitis,
STD, and TB
Prevention
6Division
of Bacterial
Diseases,
National Center
for
Immunizations
and Respiratory
Diseases
7Office
of Emergency
Preparedness and
Response,
National
Institute for
Occupational
Safety and
Health
8Council
of State and
Territorial
Epidemiologists,
Atlanta, Georgia
9Maine
Department of
Health and Human
Services,
Augusta, Maine
10National
Association of
County and City
Health
Officials,
Washington, DC
11Public
Health -- King
County, Seattle,
Washington
The material in
this report
originated in
the Coordinating
Office for
Terrorism
Preparedness and
Emergency
Response, Rich
Besser, MD,
Director; the
National Center
for
Immunizations
and Respiratory
Diseases, Anne
Schuchat, MD,
Director; the
National Center
for HIV/AIDS,
Viral Hepatitis,
STD and TB
Prevention,
Kevin A. Fenton,
MD, PhD,
Director; the
National Center
for
Preparedness,
Detection, and
Control of
Infectious
Diseases, Rima
Khabbaz, MD,
Director; the
National Center
for Injury
Prevention and
Control, Howard
Frumkin, MD,
Director,
Division of
Injury Response,
Richard Hunt,
MD, Director;
and the National
Institute for
Occupational
Safety and
Health, John
Howard, MD,
Director.
Corresponding
preparer:
Louisa Chapman,
MD, National
Center for
Immunizations
and Respiratory
Diseases, CDC,
Mailstop D-68,
1600 Clifton
Road, N.E.,
Atlanta, GA
30333.
Telephone:
404-639-8921;
Fax:
404-639-3500;
E-mail: LChapman@cdc.gov.
|