|
|
|||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||
|
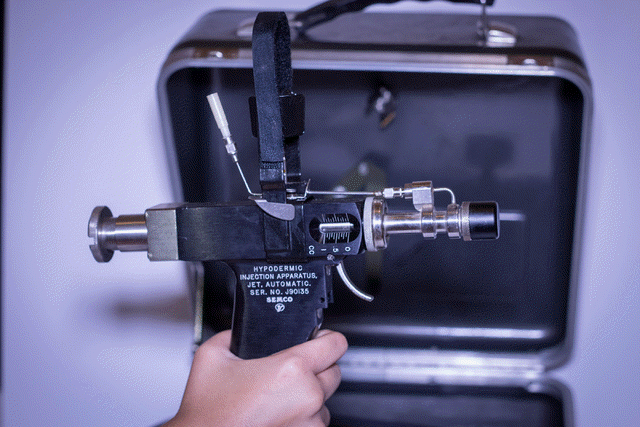
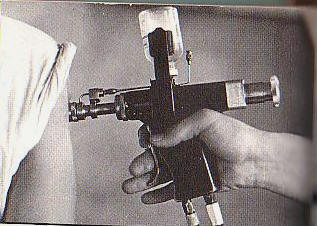
1976 Maclean’s Magazine December 27,1976 Health
Blood money: what Red Cross donors
didn’t know
A bitter behind-the-scenes struggle
between two of Canada’s most respected organizations is about to break
into the open, bringing with it at least some of the sordid details of a
murky world — the $400-million-a-year international trade in human
blood.
The Canadian Red Cross Society and
Connaught Laboratories of Toronto, which have been closely associated
for decades, are in hot dispute over which of them should process the
blood plasma the Red Cross collects from volunteer donors across the
country. It is a job Connaught has always done.
The falling-out stems from a
little-known 13-year agreement under which Connaught exported nearly
seven million dollars worth of surplus blood components and turned over
10% of the proceeds to the Red Cross, an organization that publicly
insists that human blood should neither be bought nor sold.
The Red Cross angrily contends that at
the end of 1973 Connaught exported $200,000 worth of serum albumin (a
component processed from plasma and used to treat burn victims) at a
time when Canadian hospitals were desperately short of the material.
Connaught denies the contention and claims Red cross officials had
approved the exports.
Now the Red Cross wants to sever its
relationship with Connaught, and has asked the federal and provincial to
support its plan to build a $10-million-plus fractionation (plasma
processing) plant which would be owned and operaed by the Red Cross
itself. Such a plant would severely undermine Connaught’s already
shaky financial position. Canada’s 11 governments must adjudicate,
despite their close association with both parties (the Red Cross
transfusion service receives $30-million a year from Ottawa and the
provinces; Connaught is indirectly controlled by the federal government,
through the Canada Development Corporation).
Neither party has been anxious for the
dispute to become public knowledge. Connaught has recently encountered
serious criticism on several fronts, and is jittery about its relations
with the press.
The Red Cross, fearful of offending
those Canadians who donate their blood (only 4% of the public
contributes the million pints the Red Cross collects annually), is
equally wary of controversy. "Every time, we lose donors,"
says Red Cross medical director Dr. Roger Perrault. But Perrault
believes Connaught is no longer acceptable as the processor of the
nations’ plasma, and has taken his case to the various health
minisistries.
Connaught on the other hand, vows to
fight the Red Cross plan with all the resources it can muster. "We
don’t believe it makes economic sense," says Alan Davies, the
36-year-old executive vice-president who is running Connaught on an
interim basis. "Why should the taxpayer be asked to underwrite a
new plant when we have a perfectly acceptable one here."
The Perrault proposal, however,
received tacit support at special meeting of federal and provincial
representatives in Ottawa November 23. That meeting agreed to let the
Red Cross begin a feasibility study for its project, although, as
Perrault says, "so far we have nothing on paper." The Ottawa decision was based in part on a Red Cross document that
outlined why the society wanted to end its relationship with Connaught.
The document contained many
details that cast a disturbing light on how Canada’s blood program has
been operating. It showed for instance, that donated blood components
worth $6,898,274 have been sold overseas since 1963. It revealed that roughly 40%
of a blood fraction regularly sent by the Red Cross for processing by
Connaught never returned because of contamination.
It claimed that the Red Cross is now paying Connaught double what blood
processing would cost from foreign fractionators. And it charged that
Connaught had been miserly in spending on technical improvements and
research. Davies says the Red Cross submission
was less than valid. "A lot of the Red Cross allegations are a year
and a half out of date ... we have to be terribly honest about this.
There was a time when we were having troubles with contamination, when
our yields were perhaps low ... But we have made substantial
improvements and I think I can say that our yields today compare
favorably with the best fractionators in the world." He was
distressed when told that Perrault had said: "I believe that in the
long term our differences are irreconcilable."
Replied Davies: "I do not accept
that. I cannot. Because if I accept that I’m saying essentially that
Connaught is not as interested in health care as the Red Cross is ... I
think Connaught more than most companies, has made a commitment to
health care."
The story of the Red Cross
disillusionment with Connaught has been long in the making, inextricably
mixed with the history of the national blood transfusion service itself.
The service began amid high hopes January 21, 1947, when the Red Cross
opened its first blood donor centre in Vancouver. The original aim of
the program was to eliminate commercialism. Although commercial blood
cost as much as $25 a pint, the introduction of a voluntary donor
service was not entirely based on financial grounds. Statistics showed
then, as now, that commercial blood carried a far higher risk of disease
than volunteered blood.
By 1954 the question of processing an
ever-increasing flow had to be settled. Connaught, then a University of
Toronto facility, was a research and manufacturing institute which had
been set up in 1914 to manufacture vaccines and sera. The discover of
insulin by the University of Toronto’s Frederick Banting and Charles
best in 1921, and its subsequent production gave Connaught a
well-deserved international reputation.
Connaught had produced dried blood
plasma during the Second world war and had continued to process
components into the 1950s, so it was well prepared to meet Red Cross
needs by setting up a fractionation plant in 1954. (Fractionation is the
process of splitting plasma into components that serve the same purpose
a whole blood in transfusion.) The Red Cross medical director at the time was D.
George Miller. He established the arrangements, which involved sending
Red Cross plasma to Connaught after the red cells had been removed.
Connaught then broke down the plasma into components which were returned
to the Red Cross for distribution to Canadian hospitals. For years, the
arrangement worked well — so well, in fact, that the Red
Cross decided
in 1963 to sign "a gentleman’s agreement" with Connaught for plasma work. It contained the seeds of the
current dispute. While blood technology in the rest of the world was
fast improving, Connaught’s plant was beginning to age, it’s
renowned flair for research fading.
But the Red Cross went further than
merely initiating an agreement on plasma production. For 10% of the
proceeds, it agreed to let Connaught sell certain blood components taken
from the Red Cross donors. According to Miller, the Red Cross board made
the decision on the assumption that the material would otherwise be
dumped. For 13 years, this agreement was never publicly disclosed
or even referred to in the annual reports of either organization,
although the sums involved were substantial. According to the Red Cross,
Connaught received $6,898,274 from selling blood components. The Red
cross share was $689,824.
Connaught sold some of the plasma derivatives through
its contacts. It also used a Montreal broker named Thomas Hecht, who ran
a company called Continental Pharma. His relationship with Connaught
began in the late 1950s when he sold Salk polio vaccine for the
institute. In a recent telephone interview, he said that his formal
relationship with Connaught ended in the early 1960s, although
he continued to sell blood components from Connaught on an irregular
basis into the 1970s.
During this period the world demand for blood was rising —
particularly in Europe. The large drug companies there could not get
enough suitable plasma because most European blood-donor systems were
voluntary. Private brokers began scouring the world for blood, buying it anywhere
they could, frequently from commercial "bleeding" operations
exploiting the poor in Latin America. both the International Red cross
and the World Health Organization have denounced this trade. By 1971, Hecht had made a number of sales for Connaught in Europe. He
was also involved in the selling of blood plasma not only to Europe but
also to the United States. For example, one of his executives, Victor Kubik, offered
large amounts of plasma to Warner-Lambert, the U.S. pharmaceutical
giant. Kubik protected the
source of this plasma by saying that it would not be identified by
bleeding lists. Normally, such lists are provided to show who the donors
were and where the plasma was drawn. Miller says the plasma Hecht had on offer would not have come from Red
cross donations. But, according to the Red Cross document presented in
Ottawa, the Red Cross would not have known if it had because "no
guarantee was ever given to the Red Cross, nor was any audit ever made
available from Connaught to ensure that all sales were indeed
credited." Connaught, which says it no longer does any business
with Hecht, flatly denies ever selling any whole plasma obtained from
the Red Cross. And the Red Cross says it has no reason to believe
otherwise. Nevertheless, it was the issue of whether Connaught
was selling blood components abroad without the knowledge of the Red
Cross that caused the first dispute. In early
1974,
shortly after he had taken over from the retiring Miller, Perrault
discovered that serum albumin was being sold abroad by Connaught. The discovery came when Perrault received two cheques
totaling $20,000 from Connaught. When he asked what the cheques were
for, he was told that they represented the Red Cross’ share of albumin
sales, under the long-standing
agreement.
Perrault knew of no such agreement. What he knew he
says was that there was an acute
shortage of albumin in Canada. He demanded that Connaught stop exporting
immediately, and Connaught complied. Today Connaught claims that, at the time the sales in
question were proposed and approved there was no shortage, but that soon
afterward one developed because
of a mysterious failure ("It was a freak thing, we can’t
explain it." says Davies)
Perault, on the other hand, insists there already was a
shortage that simply became more
severe when Connaught experienced the sequential batch failures.
Either way, suspicion began to replace the traditional trust.
Connaught’s regular marketing of Red Cross blood
components between 1963 and 1972 had not saved the company form its
financial troubles. (Last year it lost $1.5 million on sales of $18
million.) And while the Canada
Development Corporation’s take-over was partially a life belt
operation, Connaught’s mandate was clear: it had to become profitable
— fast.
The CDC recruited and ex-Warner-Lambert executive, Donald McCaskill as
Connaught president. McCaskill was under immediate pressure. Not only
had he to make profits with limited means, but he was saddled with
contamination problems.
Connaught processing was infecting the plasma passing through its plant
with toxins called pyrogens.
These toxins produce fever when
injected into humans.
Pyrogenic plasma is regarded in
medical circles as unfit for human
use. The pyrogenic problem at Connaught had an alarming
effect on its production of pure blood components. A Red Cross survey
showed that out of 644,745 vials of albumin due from Connaught over
a 13-year period,
fully 250,682 were 'lost'
because of contamination. In fact, the missing blood fraction was not lost.
Much of it was stored on the shelves at Connaught,
which put forward two explanations. McCaskill said, in a 1974
letter to the Red Cross,
that the "product
was discarded, written off in value, but through some unknown set of
circumstances, the physical product was retained." Davies says now that the
"blood department put it away, knowing that some day it would be
valuable." In the summer of 1973, Connaught’s
scientists began working on a process of removing pyrogens from the
tainted albumin. There were able to do this, but there was disagreement
in medico-scientific circles as to whether de-pyrogenated blood products
were fit for human use. A former vice-president of research for Connaught, Dr.
Andrew Moriarity, has said: "You can interfere with other parts of
the plasma while removing the pyrogens. It may pass tests afterward, but
you could have other faults as a result." "Despite
such reservations Connaught produced a new process within three months.
It made a confidential submission of samples of de-pyrogenated albumin
to Ottawa’s Bureau of Biologics October 19, 1973, citing the general
shortage of albumin.
On December 4, Ottawa replied. Of the three lots
submitted for testing, the assistant director of the bureau, J.L.Byrne,
wrote, two were pyrogenic, whereas your (Connaught’s) findings were
the opposite." On a controversial process that no other country then
licensed, one third of Connaught’s
sample had failed to pass. Yet Ottawa decided to release the re-processed
albumin for human use, providing
it not be released on the Canadian market without Ottawa
approval.
While this injunction was obviously intended to protect Canadians, it
placed no barrier on Connaught exporting the material.
Thirteen days after receiving the
bureau’s letter, Connaught was shipping the reprocessed albumin
abroad. The documents accompanying the exports contained no hint that
the albumin was re-processed. Otherwise it likely would have been
rejected by the importing country, which turned out to be Spain.
It was at this point that Dr. Perrault
first took office, amid growing albumin crisis in Canada. "I was
getting angry phone calls and letters from our 16 distribution centres,"
he said. "I finally wrote back assuring our people that I wasn’t
hoarding the stuff."
Perrault, a University of Ottawa medical graduate
with a Swedish PhD in blood research, knew nothing of Connaught’s
exports when he took office.
After
he found out, while grappling with a shortage in Canada, he blew up. On
April 9, 1974,
he had a confrontation with McCaskill.
The following day McCaskill
wrote to Perrault saying that in the previous November, Miller had
agreed to export the albumin and that neither of them would have allowed
it to happen had there been a shortage.
In fact, the shortage for the month of November was critical — with
the Red Cross able to fill only 884 out of 2,222 orders received. Miller
today says he has no recollection of the matter. With the exports halted, Connaught’s de-pyrogenated albumin began to
move into Canadian hospitals through the Red Cross, always after being
released by Ottawa’s Bureau of Biologics. According to an abnormal increase in patient reactions during the three
years in which treated albumin has been used in Canada,
Connaught says it regards its process as potentially very valuable, and
says it has so far refrained from publishing details because its process
cannot be patented. "We are hoping to effect an exchange of
technology with another company," Davies says.
Connaught also claims to be the only
blood fractionator in the world with a government license to distribute
de-pyrogenated material. The license, really a letter from Ottawa’s
bureau of Biologics, requires that Ottawa clear each batch as Connaught
produces it. In fact, the U.S. Bureau of Biolgics occasionally releases
for distribution material that has been de-pyrogenated by U.S.
fractionators. "But," says Dr. Hohn Finlayson of
Bethesda, Maryland, "we would naturally prefer that the processors
avoid contaminating the product in the first place."
Today Connaught claims it has largely
overcome its contamination problems and is efficient as any fractionator.
But Perrault and the Red Cross are determined to go their own way. Even
if the Red cross wins government approval for its own plant, such a
facility is at least six years away. In the meantime Perrault and the
Red Cross have to deal with Connaught, or take the politically sensitive
step of sending their plasma out of the country for processing. "I
have three offers from foreign fractionators to do our work at roughly
half what we’re paying Connaught," Perrault says. Despite their differences, Davies and Perrault are in
the midst of negotiating a formal contract to replace the
old "gentlemen’s understanding."
Perrault abrogated the 1963
agreement last March, and Davies agrees that it was the right thing to
do "We want to get on a more business-like basis," Davies
says.
Perrault adds: "I hope to negotiate a
standard fractionation contract, one that would guarantee performance
[yields] and prices." Perrault also admits that he wants to
increase the Red Cross’s share of the proceeds from any foreign sales
of surplus blood products.
"I
don’t think the 90:10 split was very fair," he says.
"Connaught was recovering far more that its costs, while were were
recovering far less." Davies says Perrault now wants 50% of the
foreign proceeds.
It all seems strange, given Connaught’s
chronic problems (the Canadian Medical Association has sent a
five-member team to study the company’s operation and the team’s
report is expected to be published in the association’s journal next
month) and given the Red Cross’s stock position that blood is too
precious to be bought or sold. But Perrault denies there is any inconsistency. "I
want to protect the volunteer donor system," he says. "I will
do anything to improve it. It doesn’t have to be done by the Red
Cross, you know. If Colgate-Palmolive can organize it better, fine...
But right now in Canada, our blood transfusion system is based on
volunteer donors and public funds. There is no room for the profit
motive. And yet the profit motive is very much in evidence. At Connaught. You’re
not supposed to make a profit on plasma, but the CDC is under orders to
make a profit. Obviously a fundamental policy
decision will have to be made."
Joseph
MacAnthony
|
|
Site Map
For problems or questions regarding
this Web site contact
|
|
|