|
|
||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||
Hepatitis C Virus Infects the Endothelial Cells of the Blood–Brain Barrier
Nicola F Fletcher, Garrick K Wilson, Jacinta Murray, Ke Hu, Andrew Lewis , Gary M Reynolds, Zania Stamataki, Luke W Meredith, Ian A Rowe, Guangxiang Luo, Miguel A Lopez-Ramirez, Thomas F Baumert, Babette Weksler, Pierre-Olivier Couraud, Kwang Sik Kim, Ignacio A Romero, Catherine Jopling, Susan Morgello, Peter Balfe and Jane A McKeating.
Gastroenterology 142:634-643, 2012. Pubmed link | Gastroenterology website | Request a preprint. Synopsis:To date, the majority of reports have studied HCV replication in hepatocytes or hepatoma-derived cells. However, HCV has been reported to replicate to low levels in non-hepatic cells, suggesting that additional cellular reservoirs exist. In this study, we demonstrate that human brain microvascular endothelium, the major component of the blood-brain barrier (BBB), expresses all major HCV entry receptors. Furthermore, two independently derived brain microvascular endothelial cell lines support HCV entry and replication providing a potential mechanism for HCV to infect the CNS. Abstract:Background & Aims: Hepatitis C Virus (HCV) infection leads to progressive liver disease and is associated with a variety of extrahepatic syndromes, including central nervous system (CNS) abnormalities. However, it is unclear whether such cognitive abnormalities are a function of systemic disease, impaired hepatic function, or virus infection of the CNS. Methods:We measured levels of HCV RNA and expression of the viral entry receptor in brain tissue samples from 10 infected individuals (and 3 uninfected individuals, as controls) and human brain microvascular endothelial cells using quantitative PCR and immunochemical and confocal imaging analyses. HCV pseudoparticles and cell culture-derived HCV were used to study the ability of endothelial cells to support viral entry and replication. Results: Using quantitative PCR, we detected HCV RNA in brain tissue of infected individuals, at significantly lower levels than in liver samples. Brain microvascular endothelia and brain endothelial cells expressed all of the recognized HCV entry receptors. Two independently derived brain endothelial cell lines, hCMEC/D3 and HBMEC, supported HCV entry and replication. These processes were inhibited by antibodies against the entry factors CD81, scavenger receptor-BI, and Claudin-1; by interferon; and by reagents that inhibit NS3 protease and NS5B polymerase. HCV infection promotes endothelial permeability and cellular apoptosis. Conclusions: Human brain endothelial cells express functional receptors that support HCV entry and replication. Virus infection of the CNS might lead to HCV-associated neuropathologies. 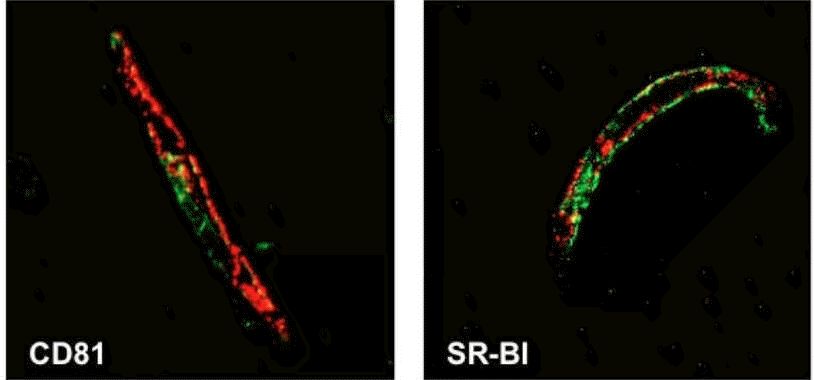 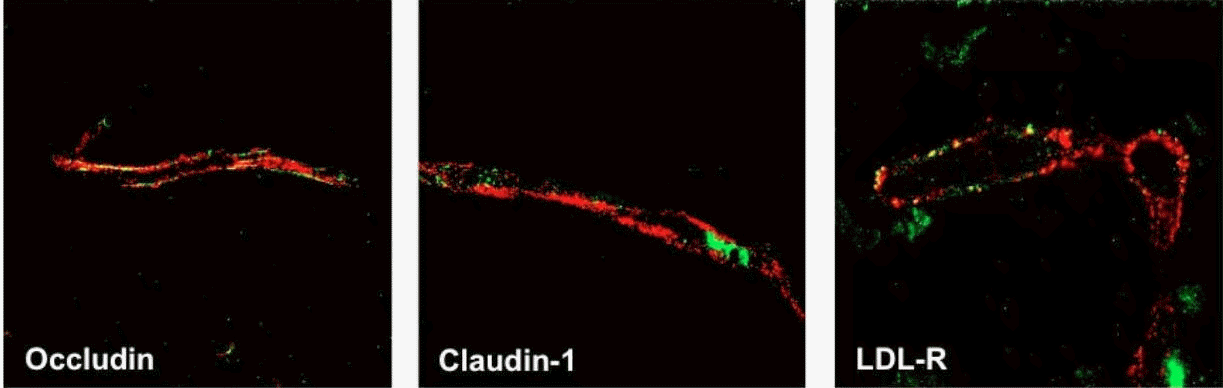 Figure 1: HCV receptor expression in human brain tissue. Formalin fixed, paraffin embedded sections of normal human brain were stained for the HCV receptors CD81, SR-BI, Claudin-1 or LDL-R (green) together with the endothelial cell-specific marker, von Willebrand Factor (vWF, red). Only brain endothelial cells express all of the receptors required for HCV entry, and in particular, SR-BI expression was restricted to endothelium. 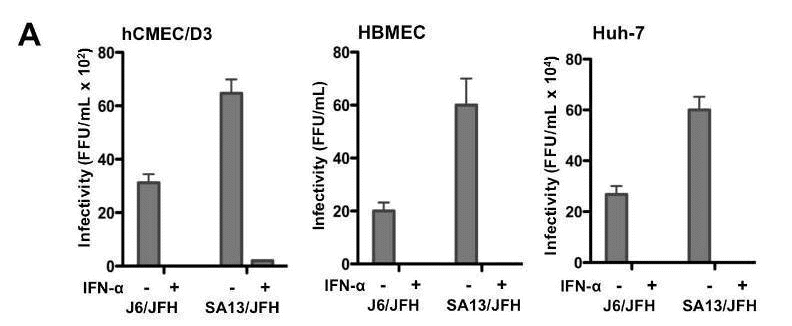
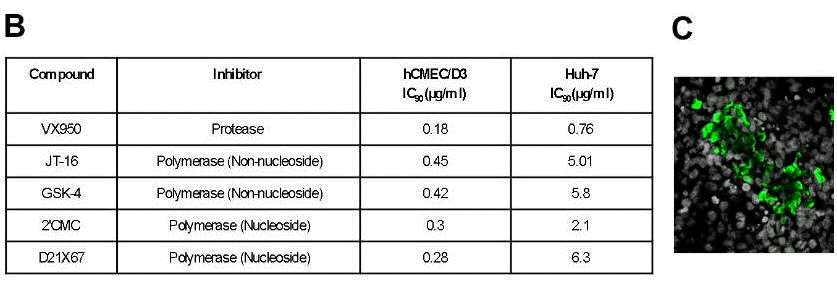
Figure 2: HCVcc infects two independently derived human brain endothelial cell lines, hCMEC/D3 and HBMEC. Two strains of HCVcc, J6/JFH and SA13/JFH, infected brain endothelial cells (A), and this was inhibited by interferon and a panel of protease and polymerase inhibitors (B). HCV infection resulted in a spreading infection of hCMEC/D3 cells (C).
 Figure 4: HCV infected brain endothelial cells release infectious virus. hCMEC/D3, Huh-7 or the non-permissive U87 cell line were incubated with HCVcc SA13/JFH at equivalent multiplicities of infection. After 12 hours, input virus was removed by extensive washing. After 72h, extracellular medium was collected and used to inoculate naïve Huh-7.5 cells. The level of infectious virus released from hCMEC/D3 over an 8 hour period was approximately 10-fold lower than that released from Huh-7 cells. No infectious virus was detected in the medium from non-permissive U87 cells. Furthermore, medium collected after just 12 hours contained no detectable virus, demonstrating that brain endothelial cells go on to release low levels of HCV that are infectious for Huh-7 hepatoma cells.
|
|
|
|