|
|
||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||
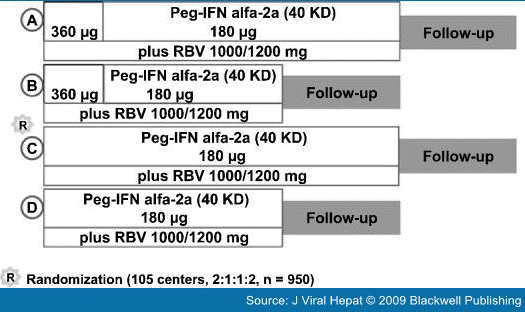
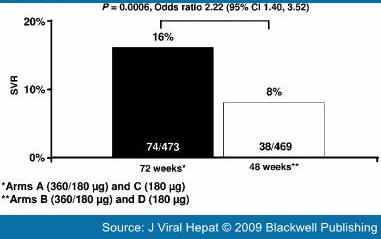
Re-treatment of Nonresponders to Conventional Interferon-based TreatmentSeveral studies have evaluated the efficacy of re-treatment with pegylated interferon plus ribavirin in previous nonresponders to conventional interferon-based therapies.[25–29,36,37] The rate of SVR is generally lower in nonresponders compared with previous relapsers. This also holds for nonresponders to previous conventional interferon plus ribavirin combination therapy than to conventional interferon monotherapy. The SVR rate in nonresponders to previous conventional interferon-based combination therapy has been reported to range from 8% to 23% after re-treatment with the standard of care.[25–29,36,37] The combination of daily interferon alfacon-1 and ribavirin has been reported to produce an SVR rate of 22% in previous nonresponders to conventional interferon plus ribavirin.[38] Re-treatment of Nonresponders to Pegylated Interferon Plus RibavirinOnly recently have studies provided insight into the effectiveness of re-treatment of patients who have not responded to combination therapy with pegylated interferon plus ribavirin. Re-treatment with standard combination regimens does not produce high SVR rates.[29,39] For example, in the international, multicenter noncomparative EPIC-3 program, the overall SVR rate was 6% in nonresponders to previous pegylated interferon (peginterferon alfa-2a or peginterferon alfa-2b) who were re-treated for 48 weeks with pegylated interferon alfa-2b (12 kD) 1.5 μg/kg per week plus ribavirin 800–1400 mg/day (Fig. 3).[29] In contrast, extending the duration of therapy in the international, randomized REPEAT trial significantly improved SVR rates in nonresponders to pegylated interferon alfa-2b (12 kD) plus ribavirin (Fig. 4). In the primary comparison, extending treatment duration to 72 weeks was significantly more effective than the standard 48-week combination regimen (Fig. 5) in prior nonresponders to at least 12 weeks of treatment with pegylated interferon alfa-2b (12 kD) plus ribavirin (16% vs 8%; odds ratio 2.22, 95% confidence interval 1.40–3.52; P = 0.0006).[39] Intensification of therapy with a 12-week, 360 μg/week fixed-dose induction regimen of peginterferon alfa-2a (40 kD) plus standard-dose ribavirin did not significantly enhance SVR rates in REPEAT. The pooled SVR rates in the induction arms of the trial (Arms A and C and D; Fig. 5) were 16% in those randomized to complete 72 weeks of treatment and 7% in those randomized to complete 48 weeks. When the two induction arms and the two noninduction arms are compared, the difference in SVR rates between the groups is 3% (13% with induction therapy vs 10% in the noninduction arms; odds ratio 0.98; 95% confidence interval 0.63–1.51). Thus, extending the duration of treatment was the strategy that produced the significant improvement in SVR rates in the two 72-week treatment groups in the trial.[39]
Figure 5.
Sustained virological response rates in previous nonresponders to pegylated interferon alfa-2b (12 kD) plus ribavirin re-treated with peginterferon alfa-2a (40 kD) 180 μg/week plus ribavirin 1000/1200 mg/day in REPEAT.[39]
The tolerability of extended treatment is an important consideration. The frequency of moderate to severe haematologic effects was broadly similar across treatment groups in REPEAT.[39] There was a slightly higher incidence of serious adverse events in patients randomized to 72 weeks than to 48 weeks of treatment (12.9% vs 10.0%) although a quantitative analysis showed that, when the probability of achieving an SVR is taken into consideration, the extended regimen has a more favourable benefit–risk ratio than the standard 48-week regimen. This is because the overall cumulative treatment duration in years required to achieve one SVR was lower in the extended treatment group (6.7 vs 10.0 in the 48 week group). Thus, the number of on-treatment serious adverse events per SVR achieved was lower in the extended treatment group compared with the standard treatment group (0.09 vs 0.15 serious adverse events per SVR achieved).[40] The combination of daily interferon alfacon-1 plus ribavirin has also been investigated in nonresponders to pegylated interferon plus ribavirin. In the DIRECT trial, which enrolled 343 patients, SVR rates of 5% and 10%, respectively, were obtained after 48 weeks of treatment with interferon alfacon 1 9 μg/day or 15 μg/day plus ribavirin 1000/1200 mg/day.[41]
|
|
|
|