|
|
||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||
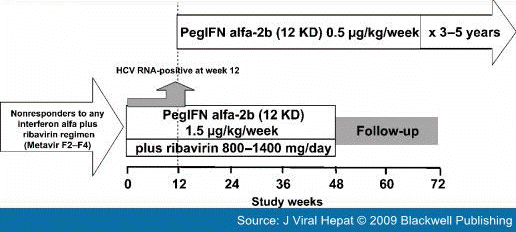
Re-treatment of RelapsersPatients who relapsed after a previous course of conventional interferon administered alone or in combination with ribavirin have a high probability of achieving an SVR when re-treated with the standard of care.[24–29] For example, in one of the largest of trials to examine re-treatment (EPIC-3, see Fig. 2 for study design), the overall SVR rate was 43% (130 of 300 patients) after re-treatment of relapsers to conventional interferon plus ribavirin with pegylated interferon alfa-2b (12 kD) 1.5 μg/kg per week plus ribavirin 800–1400 mg/day for 48 weeks.[29]
Figure 2.
Design of the multinational, noncomparative EPIC-3 program.[29] Only patients who were HCV RNA negative at week 12 were eligible to continue full-dose peginterferon alfa-2b (12 kD) plus ribavirin therapy to week 48. Patients who were HCV RNA-positive were eligible for chronic suppression therapy with low-dose peginterferon alfa-2b (12 kD) monotherapy.
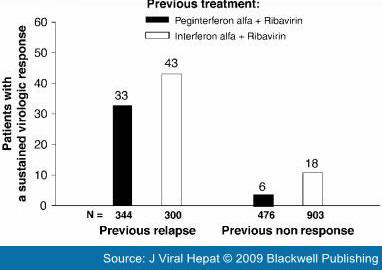
There are limited data on the efficacy of re-treatment in patients who have relapsed after treatment with pegylated interferon plus ribavirin therapy. However, patients who relapse after an inadequate duration of treatment respond well when re-treated. The overall rate of SVR was 55% after re-treatment for 48 weeks with peginterferon alfa-2a (40 kD) 180 μg/week plus ribavirin (800 mg/day or 1000/1200 mg/day) in patients who relapsed after 24 weeks of treatment with the same combination.[30] The overall SVR rate was 33% (113/344), when patients who experienced a relapse after a previous course of a pegylated interferon plus ribavirin were re-treated for 48 weeks with pegylated interferon alfa-2b (12 kD) 1.5 μg/kg per week plus ribavirin 800–1400 mg/day in the noncomparative EPIC-3 program.[29] Results from EPIC-3 are presented by previous treatment in Fig. 3.[29]
Figure 3.
Sustained virological response rates in previous relapsers and nonresponders to interferon-based therapy re-treated with pegylated interferon alfa-2b (12 kD) 1.5 μg/kg per week plus ribavirin 800–1400 mg/day in the EPIC-3 program.[29]
Patients who relapse presumably have not been exposed to a sufficient duration of treatment after HCV RNA becomes undetectable. In treatment-naïve patients with a 'slow' virological response, defined as detectable HCV RNA at weeks 4, 8 or 12, extending the duration of treatment has been shown to increase SVR rates.[31–33] From a theoretical perspective, this suggests that extending treatment to 72 weeks in previous relapsers may further improve SVR rates in this subpopulation. Preliminary data from a noncomparative German study in 107 genotype 1-infected patients who relapsed after 48 weeks of treatment with pegylated interferon plus ribavirin provide insight into the potential efficacy of this strategy.[34] When re-treated for 72 weeks with peginterferon alfa-2a (40 kD) 180 μg/week plus ribavirin 1000/1200 mg/day, the overall SVR rate was 51% (54 of 107 patients). On the basis of these data, it may be appropriate to consider extending the duration of re-treatment in relapsers, although randomized studies are needed to confirm that this strategy is effective in this setting. Few data are available on the efficacy of interferon alfacon-1 ('consensus' interferon) in patients who have relapsed after treatment with pegylated interferon plus ribavirin. An overall SVR rate of 21% was obtained in nonresponders and relapsers to pegylated interferon (alone or in combination with ribavirin) who were re-treated with daily interferon alfacon-1 (alone or in combination with ribavirin) according to a retrospective analysis of data from US Veterans Administration hospitals.[35]
|
|
|
|