|
|
||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||
|
Also see HCV Disease Dermatological side effects Dermatological adverse events (AEs) are an existing concern during hepatitis C virus (HCV) infection and peginterferon/ribavirin treatment. HCV infection leads to dermatological and muco-cutaneous manifestations including small-vessel vasculitis
Cutaneous Manifestations of Hepatitis C
Background Chronic hepatitis C infection is associated with many extrahepatic manifestations in joints, muscles, neural and gastrointestinal tissues, and skin. In this article, the many dermatologic manifestations of hepatitis C virus (HCV) are classified according to diseases with proven or suspected etiology or causation. History
Chronic hepatitis C infection occurs after the acute infection in 70% of patients with hepatitis C virus (HCV), which represents a high rate of chronicity for a viral infection. The development of chronic infection is not matched by the development of symptoms. Patients with chronic hepatitis C infection often have no symptoms for long periods.
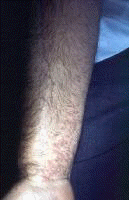
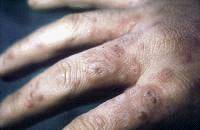
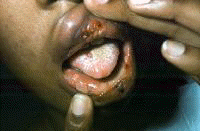
Symptoms often first develop as clinical findings of extrahepatic manifestations of HCV. In a large study of the extrahepatic manifestations of HCV, 74% of medical workers with HCV infection demonstrated extrahepatic manifestations.[16] Arthralgias (23%), paraesthesias (17%), myalgias (15%), pruritus (15%), and sicca syndrome (11%) were the most commonly occurring extrahepatic manifestations. Cryoglobulinemia was demonstrated in 50% of patients, and HCV is the most common cause of this condition. Vasculitis, arterial hypertension, purpura, LP, arthralgias, and low thyroxine levels were associated with titers positive for cryoglobulin. Biologic manifestations common to chronic hepatitis C infection include titers positive for serum cryoglobulins (40-56%), antinuclear antibody (ANA) (10%), low thyroxine (10%), and anti–smooth muscle antibody (7%). Risk factors for manifestations of extrahepatic chronic hepatitis C infection include advanced age, female sex, and liver fibrosis. Symptoms and clinical findings are most common in the joints, muscle, and skin. Arthralgias occurred in 20% of patients, skin manifestations in 17%, sicca syndrome in 23%, and sensory neuropathy in 9%.[17] Thrombocytopenia occurs in approximately 10% of patients. One or more autoantibodies frequently occur in chronic hepatitis C infection; these autoantibodies include ANA (41%), rheumatoid factor (38%), anticardiolipin antibody (27%), antithyroid antibody (13%), and anti–smooth muscle antibody (9%). One autoantibody was present in 70% of sera. Patients also present with symptoms that are less specific and are often unaccompanied by discrete dermatologic findings. Pruritus and urticaria are examples of less specific clues to underlying HCV infection in the appropriate setting (eg, posttransfusion, organ transplantation, surgery, intravenous drug use, injury of the nasal mucosa from snorting cocaine through shared straws). Patients with ongoing pathology associated with chronic hepatitis C infection that eventually results in organ failure can present with symptoms and signs in the skin. Pruritus, dryness, palmar erythema, and yellowing of the eyes and skin are examples of less specific findings in patients with end-stage liver disease with cirrhosis; these findings provide clues that lead to further evaluation of the underlying causes. Patients with the mucosa-associated lymphoma tumors (MALT) syndrome itself tend to have bowel symptoms. Chronic hepatitis C may be associated with pruritus to the extent that some authorities believe that patients with unexplained pruritus should be investigated for HCV infection.[18] Dermatologic manifestations of HCV infectionPrimary dermatologic disorders associated with chronic hepatitis C infection Lichen planus LP is a pruritic papulosquamous disorder involving the skin, scalp, nails, oral mucosa, and genitalia.[19, 20, 21, 22, 23, 24, 25, 26] The lesions and pathology are often typical unlike many papulosquamous skin disorders, such as seborrhea, atopic dermatitis, and contact dermatitis; these skin disorders have scaling, erythema, and pruritus and are included in the differential diagnosis of LP but lack its pathologic and clinical specificity. Intracellular HCV infection of epithelial cells is proven for LP.[26] Often, the papular lesions of LP suddenly appear on the volar acral surfaces of the wrists and arms and are pruritic. Oral symptoms are less common, but painful erosions can occur in oral lichen planus (OLP). Hair loss in lichen planopilaris, exquisite pruritus of markedly hypertrophic plaques on the lower legs in hypertrophic LP, and painful genital erosions can be presenting findings. The prevalence of oral, cutaneous, pharyngeal, and/or vulval LP in 87 HCV-infected patients was 19.5%.[27] Another study found no significant association between cutaneous lichen planus and HCV infection.[28] A retrospective analysis of 808 Italian patients with oral lichen planus showed that 137 of them were infected with HCV.[29] Note the images below: 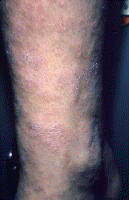 Lichen planus.  Lichen planus. 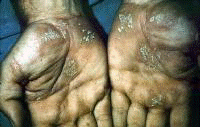 Lichen planus.
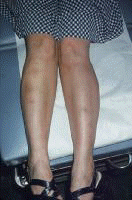
The symptomatology of acral necrolytic erythema includes pruritus associated with recurrent, erythematous, papular eruptions with blisters and erosions on the dorsal aspects of the feet and ankles.[31] Pain is common with variable-sized erosions. Chronic lesions are hyperkeratotic plaques with erosions and peripheral erythema preferring the acral parts of the legs. These lesions provide unusually specific markers for HCV infection. Sialadenitis Symptoms include dry eye and sicca syndrome due to chronic destruction of the major and minor salivary glands by capillaritis. Mooren corneal ulceration Serious corneal ulcerations are often linked to HCV infection. Cross-reactivity to the HCV envelope protein and a corneal antigen appears to be causative. Pain, lacrimation, and loss of sight result.[32] Leukocytoclastic reactions (some) This tends to appear as an eruption of palpable purpura on the lower extremities. It may represent an HCV immune complex disease. Secondary dermatologic disorders of chronic hepatitis C resulting from perturbations of the immune system Cryoglobulinemia Leukocytoclastic vasculitis occurs with type II mixed cryoglobulinemia in the skin and mucous membranes. Leukocytoclastic vasculitis also occurs with necrotizing small vessel vasculitis of the skin, kidneys, joints, and eyes. Disorders of this type belong to a group termed mixed cryoglobulinemia syndrome. These disorders display palpable purpura of the legs (which is worse distally and inferiorly), livido reticularis, ulcerations, urticaria, symmetric polyarthritis, myalgias, cutis marmorata, and fatigue. The symptomatology is pruritus, pain, or Raynaud phenomenon resulting from vasculitic sludging in the postcapillary venules of immunologic material. In all cases of cryoglobulinemia, an inflammatory reaction occurs with an influx of polymorphonuclear cells. Variable vascular damage and occlusion results in worsening degrees of pathology and symptoms; such pathology includes livido vasculitis with perturbation of superficial and deep vascular reflexes and mottling blue changes of the skin (cutis marmorata); pruritus; painful ulcerations of significant size resulting from arteriolar occlusion; and through-and-through necrosis of epidermis, dermis, and fat. Significantly, HCV localized to the intravascular debris and recently to the vessel wall establishes specificity to these reactions. Perhaps the clinical variability of vasculitic reactions is related to the degree of specificity of the vasculitis. Urticarial vasculitis with HCV infection presents with painful urticarial blanching plaques of the limbs and chest that fade to residual hyperpigmented areas.[33] The 2 cases presented lacked cryoglobulins. Complete disappearance of arthralgias and skin and liver disease occurred with clearance of HCV infection, suggesting that urticarial vasculitis may result from HCV immune complex disease. Note the images below: 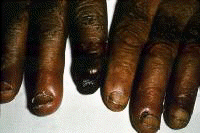 Cold agglutinin disease indistinguishable from cryoglobulinemia. Sialadenitis Dry mouth without dry eyes is the most prominent symptom of sialadenitis associated with HCV infection. Sialadenitis is an inflammatory disorder of the salivary, parotid, sublingual, and minor glands. Findings include xerostomia resulting from a chronic lymphocytic infiltrate and destruction of the salivary glands. Sjögren disease and its markers ssRo and ssLa are not found.[24, 34] As many as 15% of patients with chronic hepatitis C infections have sicca syndrome. Mooren corneal ulcer Serious corneal ulcerations begin at the rim or periphery and can progress singly or multiply and bilaterally to destroy the cornea. Symptoms of ulceration are eye pain, inflammation, tearing, and loss of vision due to corneal opacity and destruction. Antiphospholipid syndrome This is a serious multisystemic illness resulting from pathologic production of the antiphospholipids anticardiolipin and lupus anticoagulant. These IgG molecules (sometimes IgM or immunoglobulin A [IgA] in less severe variants) bind to platelets, vascular endothelium, beta-2 lipoproteins, prothrombin, and other phospholipids. The resultant pathologic processes depend on the locale of the process. Severe coagulopathies in the eye, the brain, the kidney, and large vessels result in symptomatology referable to vascular destruction or bleeding in these organs. Tertiary dermatologic disorders These are nonspecific disorders manifesting as a result of organ failure or disease of the skin associated with organ diseases. Symptoms are those of disease in the specific organs, as follows:
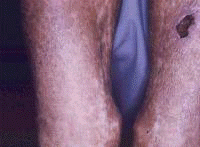
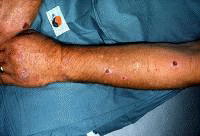
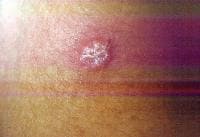
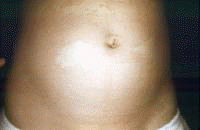
Dermatologic manifestations in which the exact pathogenesis is not further specifiedBehçet syndrome Symptoms form the major criteria for diagnosis of this disorder (see Behcet Disease). Findings for the major criteria include recurrent oral ulcerations appearing in young adults in their 20s and 30s with uveitis and genital ulcerations. Organs involved include the eyes, brain, lungs, GI tract, and kidneys. Aphthae and genital ulcerations are painful erosions. Uveitis causes pain, vision problems, and lacrimation, as well as other symptoms. Canities Graying of hair occurs with various conditions as well as naturally with aging. Prurigo (prurigo nodularis) Prurigo nodularis appears as a heaped keratotic inflammatory nodule on the extensor surfaces of the arms, legs, and trunk. Lesions are pruritic, and patients find themselves incessantly picking them with their fingers and nails. Note the images below: 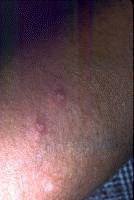 Prurigo nodularis. Lichen planus See primary dermatologic disorders for symptoms of LP. Sialadenitis Symptoms are the same as those of sicca syndrome. Thyroiditis Symptoms are manifested according to the stage of autoimmune thyroiditis. Vitiligo Symptoms are the same as those discussed in Canities, in the Physical section. Note the image below: 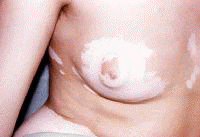 Vitiligo. Polyarteritis nodosa[35] PAN is a rare systemic vasculitis caused by necrotizing lesions in small- and medium-sized arteries of the skin, nerves, muscles, joints, kidneys, liver, and GI tract.[36] More often associated with HBV infection, PAN results from aneurysmal dilatation, hemorrhage, or ulceration in the affected artery. In PAN, false-positive results for HCV are more common than true positive results for HCV. Protean manifestations result. Major systemic symptoms of fever, malaise, and prostration occur. Clinical manifestations result from organ involvement. Skin nodulation, ulceration, and palpable purpura are common. Mononeuritis multiplex is a common presentation. Acute muscle and abdominal pain and hypertension often occur. HCV-polyarteritis nodosa represented about one fifth of HCV vasculitis in one series, tending to be severe with an acute clinical presentation yet with a good clinical remission rate.[37] Pruritus[38] Extrahepatic manifestations of HCV infection occur in 74% of patients.[16] Pruritus is one of the most common symptoms, occurring in 15% of patients. A study of HCV infection with pruritus showed that nonspecific lesions were associated with pruritus in two thirds of patients. Urticaria occurred in 5 of 29 patients, with urticarial vasculitis occurring in 1 patient. Atopic dermatitis occurred in 2 of 29 patients with HCV infection. LP was present in 4 of 29 patients, and cryoglobulinemia was present in 10 of 29 patients. Most patients in this group had xerosis and keratosis pilaris that were easily alleviated with emollients. Prurigo nodularis is more common in patients with HCV and pruritus than in other patients. Urticaria[39] and urticarial vasculitis[33] A study of 79 patients with urticaria detected anti-HCV antibody in 24% and HCV RNA in 22%. Genotype 1b was present in 71% of patients; genotype 2a, in 24%; and genotype 2b, in 6%. In patients with HCV, urticaria tends to last longer than the typical few hours, is associated with worse liver status, and leaves a brown stain. Clinical findings of urticaria are identical to those of hives. Note the images below: 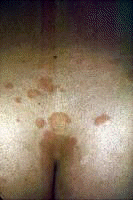 Chronic urticaria.
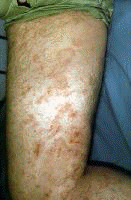
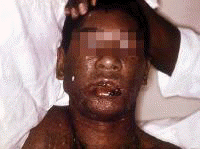
Erythema nodosum A nonspecific association, erythema nodosum (EN) has multiple causes considered in the differential diagnosis, including viral infections. EN appears as erythematous, tender, dome-shaped elevations on the skin on the lower legs. EN lesions can be single or multiple, and they can demonstrate a reaction pattern on the skin resulting from panniculitic inflammation generated by various pathologic sources (eg, infections with bacteria, mycobacteria, Protozoa, fungi, viruses). Note the images below: 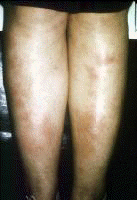 Erythema nodosa. Erythema multiforme[40] Bull's-eye lesions characterize the skin reaction pattern of erythema multiforme (EM). EM can be asymptomatic, pruritic, or burning. As an immune response to adverse antigenic stimuli from endogenous or exogenous sources, the response can be limited to a few, many, or widespread urticarial lesions with surrounding erythema and central deeply erythematous spots. Overwhelming reactions of this type include oral and anal ulcerations, systemic symptoms, and prostration; this condition is termed Stevens-Johnson syndrome. HCV infection is just one of many possible causes of EM. Note the images below: 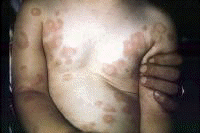 Erythema multiforme. Erythema multiforme of the oral mucosa. Erythema multiforme (Stevens-Johnson syndrome). Autoimmune thrombocytopenia Symptoms of low platelet counts occur with petechiae and purpura. Ecchymosis occurs without symptoms. Bland petechial and ecchymotic hemorrhage must be included in the differential diagnosis. Leukocytoclastic vasculitis Leukoclastic vasculitis occurs as a result of circulating immune complexed depositing with endothelial cell gaps in the skin and mucous membranes. The antigen is usually not known or determined, although it may be HCV. Dermatologic manifestations of chronic hepatitis C infection in which the cause is unknownPorphyria cutanea tarda PCT develops in patients who have 1 or more of the risk factors for PCT; the risk factors include exposure to chemical or toxic agents or drugs, iron overload, or excessive alcohol intake.[41, 42] PCT is manifested by blisters, vesicles, and milia on the acral dorsal surfaces of the extremities, especially the tops of the hands. Hypertrichosis of the temples, pigmentary changes, scarring, sclerodermatous changes, chloracne, ulcerations, and dystrophic calcifications are commonly the result of skin fragility with symptoms of epidermolysis bullosa. Uroporphyrins and hepatocarboxyl porphyrins collect in the skin, bones, and teeth after they spill into the blood once the liver is saturated. These pigments, found in the plasma, fluoresce and turn the urine dark red from renal excretion. Mild elevations in the levels of these substances and in urinary aminolevulinic acid (ALA) also occur, but porphobilinogen (PPG) levels are in the reference range. Variegate porphyria and hereditary coproporphyria have the same clinical presentation, but PPG levels are elevated in these conditions. The prevalence of PCT is related to the relative prevalence of HCV and major hemochromatosis and other iron overload genetic abnormalities. In southern Europe where HCV is prevalent, 70-90% of patients with PCT have positive results for HCV. Patients with familial PCT had negative results for HCV in one study. All patients with PCT with HCV had active multiplication of HCV. In northern Europe, Australia, and England where the prevalence of HCV is lower, 20% of patients with PCT have detectable levels of HCV. In these countries, PCT is more closely related to the higher prevalence of the hemochromatosis gene abnormality. In the United States, the prevalence of HCV in patients with PCT is intermediate at 56%. Dermatologic manifestations of malignancies associated with chronic hepatitis C diseaseNon-Hodgkin B-cell lymphoma A high prevalence (20-40%) of HCV antibodies occurs in non-Hodgkin B-cell lymphoma (NHLB), and the antibodies are not present in other lymphomas or hematologic malignancies.[43] Lymphomas producing cryoglobulins and low-grade mucosa-associated lymphoma tumors (ie, MALT syndrome) of the GI tract are associated with dermatologic manifestations. Antigen-driven B-cell proliferation from chronic stimulation is the proposed mechanism.[44, 45, 46, 47, 48] NHLB presents like other lymphomas, with lymphoidal masses, gut associated masses, and symptoms of cryoglobulinemia with palpable purpura and ulcers of the lower legs. MALT syndrome A study concerning the association of HCV and NHLB showed a prevalence of HCV of 37%.[49] Patients were older, and more patients were female than male. A closer association to immunocytoma than to MALT syndrome was found. In 20 patients with immunocytoma, 13 had HCV infection, and localization to the orbit and mucosal surfaces was more common. HCV localized to a parotid lymphoma associated with a mixed cryoglobulinemia showed viral proliferation in parotid epithelial cells and not in NHLB cells.[50] Epstein-Barr virus and herpesvirus type 6 are the other sialotropic viruses not present in the reported cases. Local carcinogenic functions of HCV, effect on the p53 system, immunoregulation perturbations, and malignant transformation were considered in the etiology of the conditions. HCV patients with B-cell lymphoma and Sjögren syndrome have a high frequency of parotid enlargement and vasculitis, an immunologic pattern overwhelmingly dominated by the presence of rheumatoid factor and mixed type II cryoglobulins, a predominance of MALT lymphomas, and an elevated frequency of primary extranodal involvement in organs in which HCV replicates (eg, exocrine glands, liver, stomach).[51] Hepatoma The progression of HCV infection to chronic hepatitis C infection and then to hepatocellular carcinoma is a well-known sequence. The chronicity of HCV infection occurs in 60% of patients infected by the virus overall, but the rate increases to more than 90% in type 1b infection.[3] Worse hepatocellular disease also resulted from type 1b infections. Hepatic cirrhosis is correlated with inoculation size, being more common in patients with HCV-infected blood transfusions (23%) and organ transplantation than in users of intravenous drugs (9%). Viral species variation, complications, and host antibody response vigor have a role in chronic infection.[52] Cirrhosis develops in approximately 15-20% of patients with chronic hepatitis C. Hepatocellular carcinoma eventually occurs in 5-10% of patients with chronic hepatitis C. Symptoms of hepatoma include rapid decompensation of the liver and metastases, often to the brain. Squamous cell carcinoma of the tongue This condition is a rarely coexistent disorder, usually evident as an erosion on the tongue. Dermatologic manifestations associated with interferon alfa therapy for HCV infectionErosive OLP and epidermolytic hyperkeratosis may occur with interferon alfa therapy for chronic hepatitis C infection.[53] Capillaritis may be present with interferon alfa treatment of chronic hepatitis C infection.[54] Lichen myxedematosus may worsen during interferon alfa-2a therapy for chronic active hepatitis. Finally, thyroiditis may occur during interferon therapy. Possible conditions associated with HCV infectionErythema dyschromicum perstans is an asymptomatic condition of ashy gray discoloration of the skin on the extensor surfaces of the arms and face. Porokeratosis, the disseminated superficial type, has been associated with HCV infection or hepatocellular carcinoma. Eruptive disseminated porokeratosis in relation to the onset of hepatocellular carcinoma occurred in 3 closely observed patients with chronic hepatitis C infection and persistent HCV hepatitis. Both conditions are believed to be related to immunomodulation of the TP53 gene. HCV core protein may affect cancer transformation directly through an effect on a promoter gene expression.[55] The core protein is a multifunctional protein with the capacity to bind to the so-called death domain of tumor necrosis factor receptor 1 (TNFR1) and the intracellular portion of lymphotoxin-beta receptor. The portion of TNFR1 active in apoptosis and antiapoptosis signaling pathway is the death domain affected by HCV.[56] Generalized granuloma annulare, an asymptomatic condition with dermal nodule formation resulting from an inflammation of collagen, may be present.[57] Symmetric polyarthritis and livido reticularis may occur.[58] Asymptomatic, solitary, dome-shaped reddish papules, 5-10 mm in diameter, may be present on the face in some patients with HCV infection; these papules represent arteriovenous hemangiomas, which are seen with increased frequency in these patients. Progressive pigmented purpura (Gougerot-Blum disease) is demonstrated as lesions of capillaritis on the lower legs with fine petechiae in various distributions.[59] A possible association between erythema induratum (nodular vasculitis) and HCV infection has been suggested.[60] Sarcoidosis may occur in HCV patients, especially in association with antiviral immunotherapy.[61] Physical
Physical findings of extrahepatic dermatologic conditions associated with hepatitis C virus (HCV) infection have largely been presented in History. Physical findings of the disorders and diseases are clarified below.
Primary dermatologic disorders of chronic hepatitis C in which HCV infection of the skin is proven or suggestedLP presents as flat-topped pruritic papules or coalescent plaques on the extensor surfaces of the limbs and trunk. Some individual lesions may have a purple-red hue, a fine retiform surface scale (termed Wickham striae), and an angular appearance. Involvement of the scalp hair is a cause of alopecia of the scarring variety (termed lichen planopilaris). The leaf-shaped alopecic areas appear like fingertips touching the scalp with normal intervening areas. Oral involvement with buccal lesions is common. Gum, lip, and tongue involvement can be a chronic premalignant disorder. Lesions lack the specificity of skin lesions and present as purple-red mucosal patches. Occasionally, prurigolike nodules with heaped keratotic material on a thickened base occur on the limbs and trunk. Lesions on the genitalia can be nonspecific and are one cause of vulvar pruritus. The lesions of acral necrolytic erythema are flaccid bullae and described as large, painful, partially eroded, violaceous patches on the proximal half of the dorsal aspect of the feet extending over the medial and lateral malleolus.[30] Some leukocytoclastic reactions may occur, described as follows:
Sialadenitis associated with HCV infection can present as a sicca syndrome with dry eyes and mouth and ulcerations from destruction of the salivary and meibomian glands. Secondary dermatologic disorders of chronic hepatitis C infection (mostly as a result of perturbations of the immune system)Cryoglobulinemia with leukocytoclastic and necrotizing small vessel vasculitis of the skin, kidneys, joints, and eyes. Physical findings in the skin include palpable purpura and ulcerations on the lower legs. Sialadenitis presents as dry eyes and dry oral and nasal mucosa. Ulcerations occur late in the evolution of this disorder. In Mooren corneal ulcer, corneal ulcers form on one or both eyes beginning at the periphery or limbus. Antiphospholipid syndrome results in protean physical findings in the brain, kidneys, eyes, skin, joints, and major vessels because of thrombus formation and bleeding in these major organs. Autoimmune factors, not further categorized, are as follows:
Tertiary dermatologic disorders (nonspecific disorders manifested because of organ failure or because disease is manifested in the skin)Physical findings of liver failure result from cirrhosis, autoimmune hepatitis, cholangitis, and hepatocellular carcinoma. Findings include ascites, jaundice, upper GI tract bleeding, and various accompaniments of chronic and acute liver decompensation. Physical findings of hypothyroidism include coarsened and thickened dry hair and skin and myxedematous plaques on the shins (see Hypothyroidism). Dermatologic manifestations of HCV infection in which the cause is uncertain but the association is certainPCT is manifested by blisters, vesicles, and milia on the acral dorsal surfaces of the extremities, especially the tops of the hands. Often, hypertrichosis of the temples, pigmentary changes, scarring, sclerodermatous changes, and chloracne are present, and ulcerations with dystrophic calcifications result from skin fragility. Dermatologic manifestations of malignancies associated with chronic hepatitis C diseaseLike all lymphomas, NHL appears as nodal masses with physical signs referable to the area where they develop and disrupt. Clinical findings of verrucous squamous cell carcinoma of the tongue include a thickened patch in OLP on the tongue.[63] A possible relationship exists to altered TP53 gene transformation in squamous cell carcinoma and hepatoma in cirrhosis. MALT syndrome appears as mass lesions in the orbit or in a gut-associated area (eg, salivary gland or intra-abdominal, hepatic, or other area). Hepatoma physically appears as a mass lesion in the liver; a metastatic lesion, often to the brain or lung; and metabolic disruptions of those organs. Dermatologic manifestations of treatments for HCV infectionLichen myxedematosus may worsen during interferon alfa-2a therapy for chronic active hepatitis.[64] Asymptomatic, 2- to 4-mm, flesh-colored papules on the upper and lower extensor surfaces of the arms are noted. Lichen myxedematosus papules contain depositions of mucin in patients without thyroid disease who often have paraproteinemias. A localized form affects the head and neck, upper limbs, lower limbs, or trunk. A generalized form is termed scleromyxedema and affects large areas with sclerosis. Capillaritis is associated with interferon alfa treatment of HCV infection.[54] Pigmented purpuric eruptions on the lower legs consist of fine petechiae that appear after beginning treatment. Erosive OLP and epidermolytic hyperkeratosis may occur during interferon alfa-2b therapy for chronic hepatitis C infection. These conditions appear with erythematous, scaly plaques on the scalp, chest, back, buttocks, legs, and genitalia and painful ulcerations in the mouth. The skin lesions are psoriasiform with a reddened base and variable heaped-up scale. Wickham striae occur on the lips and intraorally.[53] Possible associationsSome patients with HCV infection have an asymptomatic, solitary, 5- to 10-mm diameter, dome-shaped reddish papule on the face, which represents arteriovenous hemangioma and occurs with increased frequency. Gougerot-Blum disease is a manifestation of HCV infection.[59] Lesions of capillaritis on the lower legs are noted with fine petechiae in various distributions. Causes
The causes of extrahepatic dermatologic manifestations of hepatitis C virus (HCV) infection relate to the nature of the virus, the method of infection, host responses to HCV infection, and myriad feedback considerations. HCV is a flavivirus with a positive sense single-strand RNA (ssRNA); the ends of the RNA are conserved. HCV RNA encodes 3300 amino acids. The polyprotein encoded is cleaved by host and viral proteases to yield at least 9 polypeptide proteins, core peptides, and viral envelope proteins E1 and E2 (which encode for glycoprotein spikes set in the host cell membrane derived envelope).
Six nonstructural (NS) proteins, termed NS2, NS3, NS4a, NS4b, NS5a, and NS5b, include RNA polymerase, cyclase, and other proteins necessary for viral replication. Hypervariable regions of the E2 envelope protein are responsible for quasi-species generation. Some nonessential sequences of NS genes also create variability in antigenic structure. Mutation of NS3 sections results in escape mutants that conserve essential viral functions while interfering with host T-cell function by down-regulating interleukin 2 and interferon gamma function and up-regulating interleukin 10.[46] The chronicity of infection also relates to inoculum size, with high rates and worse disease in patients who have undergone transfusions and organ transplantation. The resulting persistent infection and immune stimulation create the many immunologic epiphenomenon, such as LP, mixed cryoglobulinemia, and thyroiditis. A generation of factors unfavorable to apoptosis control functions may favor malignant transformation in chronic hepatitis C infection. Primary dermatologic disorders of chronic hepatitis C infectionLichen planus HCV particles are found in immune lymphocytes, macrophages, and dendritic cells, as well as in epithelial cells and cells of blood vessels.[26, 65, 66] In LP, HCV replicates in OLP epithelial cells of lesional and nonlesional skin cells. What role this plays in developing the LP host response is not known. HCV perturbs the class II host response by impairing the ability of the dendritic or antigen-presenting cell to stimulate a good T-cell response.[67] Lower expression of interferon gamma and interleukin 12 causes a blunted T-cell response to allostimulatory non–self-related new antigen. Another mechanism of class II immune response inhibition results from the hypervariable region (HVR-1) of envelope protein E2 suppressing CD4+ lymphocytes, which are stimulated to respond to HCV. The HVR-1 acts as a T-cell receptor antagonist.[68] A theory of causation, termed the superantigen theory of immune stimulation (which includes LP and many other immune-related disorders in which exact causation is a mystery), suggests that the human leukocyte antigen (HLA) class II response was disturbed. Superantigens, such as bacterial proteins and toxins, were proposed to act outside the HLA binding site to broadly stimulate an immune nonspecific response of wide varieties of T cells, including antiself immune responses. Some leukocytoclastic reactions Type II cryoglobulinemias in which IgM is the macromolecular rheumatoid antibody are more common than type III cryoglobulinemias. IgG was directed toward NS proteins and structural proteins. IgM antigen was the core protein, suggesting cross-reactivity to IgG.[69] Co-affinity of IgM anticore antibody to IgG may be a mechanism in the formation of the cryoprecipitate. Sialadenitis This condition is a capillaritis of the accessory and main salivary glands and the eyes. Secondary dermatologic disorders of chronic hepatitis C infection (mostly as a result of perturbations of the immune systemCryoglobulinemia Leukocytoclastic vasculitis of the skin, kidneys, joints, and eyes is present. Immune stimulation of T-cell clones in HCV infection produces monoclonal macroglobulins with co-affinity to a constituent of HCV and IgG. Stimulation of this rheumatoid factor is driven by anti-HCV activity sustained by the ongoing mutation of the virus. The same pathogenesis is involved for NHL associated with HCV. The mean cryoprecipitate result was 2% in one study[69] but can be greater. Immunoglobulin M (IgM) directed to a core protein sequence of HCV was also directed to a homologous immunoglobulin G (IgG) heavy-chain amino acid sequence. IgG was directed to structural and nonstructural components of HCV. The material is usually monomeric IgM molecules with high affinity for IgG (also termed rheumatoid IgM molecules or rheumatoid factor). Type I cryoglobulinemias are caused by macroglobulins, such as in Waldenström macroglobulinemia, or IgG in which sludging results from massive molecules congealing in cooled venules of the lower leg. Type II mixed cryoglobulins have a monoclonal rheumatoid species but vary in the IgG antigen, which is nonspecific. Type III mixed cryoglobulinemias result from specific reactions of immunoglobulins to a single or well-defined antigen or various antigens, but the antibody species and the antigen are not monoclonal. HCV-related systemic vasculitis may also occur in the absence of detectable mixed cryoglobulinemia.[70] Sialadenitis This condition can result from acute bacterial or viral infection or autoimmune disease, as in Sjögren disease. Mooren corneal ulcer Cross-reactivity to HCV envelope protein and a corneal antigen appears to be causative. A HCV envelope protein shares the antigenic specificity of this protein, resulting in Mooren ulcer in HCV infection. Other common etiologic causes may occur. Antiphospholipid syndrome Immune reactant immunoglobulins with antiphospholipid activity are present. Autoimmune, not further categorized Note the following:
Tertiary dermatologic disorders (nonspecific disorders manifesting because of organ failure or because disease manifests in the skin)Persistent viral particles, persistent HCV hepatitis, and transformation are promoted by means already stated. Dermatologic manifestations of HCV infection in which the cause is uncertain but the association is certainProposed etiologic mechanisms for the relationship of chronic hepatitis C infection and PCT include oxidative stress from chronic hepatitis C and other viral infections (HBV and HIV) of hepatocytes that affect uroporphyrin decarboxylase (UDC) function. HIV infection causes increased serum porphyrin levels.[71] Patients with hereditary PCT and patients with hemochromatosis have sufficient UDC redundant function to prevent the appearance of symptoms. Cytochrome P4501A2, metabolically active iron, chronic hepatitis C, long-term alcohol intake, and estrogens affect the rate of conversion of uroporphyrinogen to uroporphyrin by oxidation in hepatocytes. An increase in delta5-ALA synthetase activity can present excess uroporphyrin to the hepatocyte. The clinical and immunologic pattern of expression of Sjögren syndrome associated with chronic HCV infection was analyzed.[72] HCV-associated Sjögren syndrome is indistinguishable from the primary form in most cases. Chronic HCV infection was suggested as an exclusion criterion for the classification of primary Sjögren syndrome because the virus may be implicated in the development of Sjögren syndrome in a specific subset of patients. The phrase "Sjögren syndrome secondary to HCV" was recommended. Dermatologic manifestations of malignancies associated with chronic hepatitis C diseaseNon-Hodgkin lymphoma A protein amino acid sequence of HCV core protein has homology to a heavy-chain IgG sequence. The viral recognition reaction producing macroglobulin rheumatoid species antibodies may drive the mixed cryoglobulinemia reaction, and it may also cause lymphomagenesis. Verrucous cell carcinoma of the tongue and OLP with HCV infection and replication A possible relationship to altered TP53 gene transformation exists with squamous cell carcinoma and hepatoma in cirrhosis. MALT syndrome A study concerning the association of HCV and NHLB showed a prevalence of HCV of 37%.[49] Patients were older, and more patients were female than male. A closer association to immunocytoma was found than to MALT syndrome. Thirteen of 20 cases of immunocytoma had HCV infection, and localization to the orbit and mucosal surfaces was more common. HCV localized to a parotid lymphoma associated with a mixed cryoglobulinemia showed viral proliferation in parotid epithelial cells and not in NHLB cells.[50] Epstein-Barr virus and herpesvirus type 5 are the other sialotropic viruses not present in the reported cases. Local carcinogenic functions of HCV, effect on the p53 system, immunoregulation, perturbations, and malignant transformation were considered in the etiology of the conditions. Hepatoma The ends of HCV RNA are conserved. HCV core protein is a multifunctional protein with a role in apoptosis-antiapoptosis regulation of cell multiplication and tumor control.[56] The death domain of tumor necrosis factor 1 and the cytoplasmic tail of lymphotoxin beta bind to the core protein. Nuclear factor kappa B (NF-kB) is activated in core peptide-transfected hepatoma cells and in HCV-infected liver tissue. NF-kB activation causes resistance to apoptotic signals in transfected HCV-containing cells. An evasion mechanism of host surveillance by means of NF-kB activation is proposed as one method of viral persistence in the chronic hepatitis C state and in carcinogenesis. Dermatologic manifestations of treatments for HCV infectionInterferon therapy increases the incidence of immune epiphenomenon and autoimmune disorders. Differentials
Contributor
Information and
Disclosures 1. Sterling RK, Bralow S. Extrahepatic manifestations of hepatitis C virus. Curr Gastroenterol Rep. Feb 2006;8(1):53-9. [Medline]. 2. Maticic M. Lichen planus in hepatitis C virus infection: an early marker that may save lives. Acta Dermatovenerol Alp Panonica Adriat. Mar 2007;16(1):3-6. [Medline]. 3. Bonkovsky HL, Mehta S. Hepatitis C: a review and update. J Am Acad Dermatol. Feb 2001;44(2):159-82. [Medline]. 4. Alter MJ, Kruszon-Moran D, Nainan OV, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. Aug 19 1999;341(8):556-62. [Medline]. 5. Hepatitis C: global prevalence. Wkly Epidemiol Rec. Nov 14 1997;72(46):341-4. [Medline]. 6. Gordon SC, Bayati N, Silverman AL. Clinical outcome of hepatitis C as a function of mode of transmission. Hepatology. Aug 1998;28(2):562-7. [Medline]. 7. Hu KQ, Tong MJ. The long-term outcomes of patients with compensated hepatitis C virus-related cirrhosis and history of parenteral exposure in the United States. Hepatology. Apr 1999;29(4):1311-6. [Medline]. 8. Fattovich G, Giustina G, Degos F, et al. Effectiveness of interferon alfa on incidence of hepatocellular carcinoma and decompensation in cirrhosis type C. European Concerted Action on Viral Hepatitis (EUROHEP). J Hepatol. Jul 1997;27(1):201-5. [Medline]. 9. Seeff LB, Miller RN, Rabkin CS, et al. 45-year follow-up of hepatitis C virus infection in healthy young adults. Ann Intern Med. Jan 18 2000;132(2):105-11. [Medline]. 10. Vogt M, Lang T, Frosner G, et al. Prevalence and clinical outcome of hepatitis C infection in children who underwent cardiac surgery before the implementation of blood-donor screening. N Engl J Med. Sep 16 1999;341(12):866-70. [Medline]. 11. Obando J, Tororelli K, Banner B. Iron: the major HFE gene mutation and chronic hepatitis C [abstract]. Gastroenterology. 1999;118:A593. 12. Banner BF, Karamitsios N, Smith L, Bonkovsky HL. Enhanced phenotypic expression of alpha-1-antitrypsin deficiency in an MZ heterozygote with chronic hepatitis C. Am J Gastroenterol. Sep 1998;93(9):1541-5. [Medline]. 13. Nakashima K, Ikematsu H, Hayashi J, Kishihara Y, Mutsutake A, Kashiwagi S. Intrafamilial transmission of hepatitis-C virus among the population of an endemic area of Japan. JAMA. Nov 8 1995;274(18):1459-61. [Medline]. 14. Osella AR, Misciagna G, Leone A, Di Leo A, Fiore G. Epidemiology of hepatitis C virus infection in an area of Southern Italy. J Hepatol. Jul 1997;27(1):30-5. [Medline]. 15. Kiyosawa K, Tanaka E, Sodeyama T, et al. Transmission of hepatitis C in an isolated area in Japan: community-acquired infection. The South Kiso Hepatitis Study Group. Gastroenterology. Jun 1994;106(6):1596-602. [Medline]. 16. Cacoub P, Poynard T, Ghillani P, et al. Extrahepatic manifestations of chronic hepatitis C. MULTIVIRC Group. Multidepartment Virus C. Arthritis Rheum. Oct 1999;42(10):2204-12. [Medline]. 17. Cacoub P, Renou C, Rosenthal E, Cohen P, Loury I, Loustaud-Ratti V. Extrahepatic manifestations associated with hepatitis C virus infection. A prospective multicenter study of 321 patients. The GERMIVIC. Groupe d Etude et de Recherche en Medecine Interne et Maladies Infectieuses sur le Virus de l Hepatite C. Medicine (Baltimore). Jan 2000;79(1):47-56. [Medline]. 18. Dervis E, Serez K. The prevalence of dermatologic manifestations related to chronic hepatitis C virus infection in a study from a single center in Turkey. Acta Dermatovenerol Alp Panonica Adriat. Sep 2005;14(3):93-8. [Medline]. 19. Chuang TY, Stitle L, Brashear R, Lewis C. Hepatitis C virus and lichen planus: A case-control study of 340 patients. J Am Acad Dermatol. Nov 1999;41(5 Pt 1):787-9. [Medline]. 20. Nagao Y, Sata M, Kage M, Kameyama T, Ueno T. Histopathological and immunohistochemical study of oral lichen planus-associated HCV infection. Eur J Intern Med. Oct 2000;11(5):277-282. [Medline]. 21. Nagao Y, Sata M, Fukuizumi K, Ryu F, Ueno T. High incidence of oral lichen planus in an HCV hyperendemic area. Gastroenterology. Sep 2000;119(3):882-3. [Medline]. 22. Parodi A, Divano MC, Rebora A. Serologic markers of autoimmune chronic hepatitis in patients with lichen planus. Eur J Dermatol. 1996;6:30-1. 23. Sanchez-Perez J, De Castro M, Buezo GF, Fernandez-Herrera J, Borque MJ, Garcia-Díez A. Lichen planus and hepatitis C virus: prevalence and clinical presentation of patients with lichen planus and hepatitis C virus infection. Br J Dermatol. Apr 1996;134(4):715-9. [Medline]. 24. Mignogna MD, Lo Muzio L, Lo Russo L, Fedele S, Ruoppo E, Bucci E. Oral lichen planus: different clinical features in HCV-positive and HCV-negative patients. Int J Dermatol. Feb 2000;39(2):134-9. [Medline]. 25. Mignogna MD, Lo Muzio L, Favia G, Mignogna RE, Carbone R, Bucci E. Oral lichen planus and HCV infection: a clinical evaluation of 263 cases. Int J Dermatol. Aug 1998;37(8):575-8. [Medline]. 26. Arrieta JJ, Rodriguez-Inigo E, Casqueiro M, et al. Detection of hepatitis C virus replication by In situ hybridization in epithelial cells of anti-hepatitis C virus-positive patients with and without oral lichen planus. Hepatology. Jul 2000;32(1):97-103. [Medline]. 27. Nagao Y, Kawasaki K, Sata M. Insulin resistance and lichen planus in patients with HCV-infectious liver diseases. J Gastroenterol Hepatol. Apr 2008;23(4):580-5. [Medline]. 28. Maticic M, Poljak M, Lunder T, Rener-Sitar K, Stojanovic L. Lichen planus and other cutaneous manifestations in chronic hepatitis C: pre- and post-interferon-based treatment prevalence vary in a cohort of patients from low hepatitis C virus endemic area. J Eur Acad Dermatol Venereol. Jul 2008;22(7):779-88. [Medline]. 29. Carbone M, Arduino PG, Carrozzo M, et al. Course of oral lichen planus: a retrospective study of 808 northern Italian patients. Oral Dis. Apr 2009;15(3):235-43. [Medline]. 30. Khanna VJ, Shieh S, Benjamin J, et al. Necrolytic acral erythema associated with hepatitis C: effective treatment with interferon alfa and zinc. Arch Dermatol. Jun 2000;136(6):755-7. [Medline]. 31. Halpern AV, Peikin SR, Ferzli P, Heymann WR. Necrolytic acral erythema: an expanding spectrum. Cutis. Dec 2009;84(6):301-4. [Medline]. 32. Monroe LR. Fatal sight: Mooren's ulcer. EyeNet Magazine. April 1998;2(4). 33. Hamid S, Cruz PD Jr, Lee WM. Urticarial vasculitis caused by hepatitis C virus infection: response to interferon alfa therapy. J Am Acad Dermatol. Aug 1998;39(2 Pt 1):278-80. [Medline]. 34. Haddad J, Deny P, Munz-Gotheil C, et al. Lymphocytic sialadenitis of Sjögren's syndrome associated with chronic hepatitis C virus liver disease. Lancet. Feb 8 1992;339(8789):321-3. [Medline]. 35. Domingo P, Ris J, Martinez E, Casas F. Erythema nodosum and hepatitis C. Lancet. Dec 1 1990;336(8727):1377. [Medline]. 36. Morgan AJ, Schwartz RA. Cutaneous polyarteritis nodosa: a comprehensive review. Int J Dermatol. Jul 2010;49(7):750-6. [Medline]. 37. Saadoun D, Terrier B, Semoun O, et al. Hepatitis C virus-associated polyarteritis nodosa. Arthritis Care Res (Hoboken). Oct 27 2010;[Medline]. 38. Davis GL, Esteban-Mur R, Rustgi V, et al. Interferon alfa-2b alone or in combination with ribavirin for the treatment of relapse of chronic hepatitis C. International Hepatitis Interventional Therapy Group. N Engl J Med. Nov 19 1998;339(21):1493-9. [Medline]. 39. Reichel M, Mauro TM. Urticaria and hepatitis C. Lancet. Sep 29 1990;336(8718):822-3. [Medline]. 40. Neri S, Raciti C, D'Angelo G, Ierna D, Bruno CM. Hyde's prurigo nodularis and chronic HCV hepatitis. J Hepatol. Jan 1998;28(1):161-4. [Medline]. 41. Bulaj ZJ, Phillips JD, Ajioka RS, et al. Hemochromatosis genes and other factors contributing to the pathogenesis of porphyria cutanea tarda. Blood. Mar 1 2000;95(5):1565-71. [Medline]. 42. Cribier B, Petiau P, Keller F, et al. Porphyria cutanea tarda and hepatitis C viral infection. A clinical and virologic study. Arch Dermatol. Jul 1995;131(7):801-4. [Medline]. 43. Luppi M, Longo G, Ferrari MG, et al. Clinico-pathological characterization of hepatitis C virus-related B-cell non-Hodgkin's lymphomas without symptomatic cryoglobulinemia. Ann Oncol. May 1998;9(5):495-8. [Medline]. 44. De Re V, De Vita S, Marzotto A, et al. Sequence analysis of the immunoglobulin antigen receptor of hepatitis C virus-associated non-Hodgkin lymphomas suggests that the malignant cells are derived from the rheumatoid factor-producing cells that occur mainly in type II cryoglobulinemia. Blood. Nov 15 2000;96(10):3578-84. [Medline]. 45. Domingo JM, Romero S, Moreno JA, Domingo JA, Callen L, Gutierrez M. Hepatitis C virus infection and mixed cryoglobulinemia in patients with lymphoproliferative diseases. Haematologica. Jan 1999;84(1):94-6. [Medline]. 46. Wang H, Eckels DD. Mutations in immunodominant T cell epitopes derived from the nonstructural 3 protein of hepatitis C virus have the potential for generating escape variants that may have important consequences for T cell recognition. J Immunol. Apr 1 1999;162(7):4177-83. [Medline]. 47. Guastafierro S, Sessa F, Tirelli A. Biclonal gammopathy and platelet antibodies in a patient with chronic hepatitis C virus infection and mixed cryoglobulinemia. Ann Hematol. Aug 2000;79(8):463-4. [Medline]. 48. Chang KM, Gruener NH, Southwood S, et al. Identification of HLA-A3 and -B7-restricted CTL response to hepatitis C virus in patients with acute and chronic hepatitis C. J Immunol. Jan 15 1999;162(2):1156-64. [Medline]. 49. Vallisa D, Berte R, Rocca A, et al. Association between hepatitis C virus and non-Hodgkin's lymphoma, and effects of viral infection on histologic subtype and clinical course. Am J Med. May 1999;106(5):556-60. [Medline]. 50. De Vita S, Sansonno D, Dolcetti R, et al. Hepatitis C virus within a malignant lymphoma lesion in the course of type II mixed cryoglobulinemia. Blood. Sep 1 1995;86(5):1887-92. [Medline]. 51. Ramos-Casals M, la Civita L, de Vita S, et al. Characterization of B cell lymphoma in patients with Sjögren's syndrome and hepatitis C virus infection. Arthritis Rheum. Feb 15 2007;57(1):161-70. [Medline]. 52. Villano SA, Vlahov D, Nelson KE, Cohn S, Thomas DL. Persistence of viremia and the importance of long-term follow-up after acute hepatitis C infection. Hepatology. Mar 1999;29(3):908-14. [Medline]. 53. Schlesinger TE, Camisa C, Gay JD, Bergfeld WF. Oral erosive lichen planus with epidermolytic hyperkeratosis during interferon alfa-2b therapy for chronic hepatitis C virus infection. J Am Acad Dermatol. Jun 1997;36(6 Pt 1):1023-5. [Medline]. 54. Gupta G, Holmes SC, Spence E, Mills PR. Capillaritis associated with interferon-alfa treatment of chronic hepatitis C infection. J Am Acad Dermatol. Nov 2000;43(5 Pt 2):937-8. [Medline]. 55. Kono T, Kobayashi H, Ishii M, Nishiguchi S, Taniguchi S. Synchronous development of disseminated superficial porokeratosis and hepatitis C virus-related hepatocellular carcinoma. J Am Acad Dermatol. Nov 2000;43(5 Pt 2):966-8. [Medline]. 56. Tai DI, Tsai SL, Chen YM, et al. Activation of nuclear factor kappaB in hepatitis C virus infection: implications for pathogenesis and hepatocarcinogenesis. Hepatology. Mar 2000;31(3):656-64. [Medline]. 57. Granel B, Serratrice J, Rey J, et al. Chronic hepatitis C virus infection associated with a generalized granuloma annulare. J Am Acad Dermatol. Nov 2000;43(5 Pt 2):918-9. [Medline]. 58. Shearer CM, Jackson JM, Callen JP. Symmetric polyarthritis with livedo reticularis: a newly recognized manifestation of hepatitis C virus infection. J Am Acad Dermatol. Oct 1997;37(4):659-61. [Medline]. 59. Chima N, Houghton JP, Igwegbe I, et al. Gougerot-Blum disease as a manifestation of hepatitis C infection. J Cutan Pathol. 2000;27:569. 60. Fernandes SS, Carvalho J, Leite S, et al. Erythema induratum and chronic hepatitis C infection. J Clin Virol. Apr 2009;44(4):333-6. [Medline]. 61. Faurie P, Broussolle C, Zoulim F, Trepo C, Sève P. Sarcoidosis and hepatitis C: clinical description of 11 cases. Eur J Gastroenterol Hepatol. Mar 6 2010;[Medline]. 62. Jackson JM, Callen JP. Scarring alopecia and sclerodermatous changes of the scalp in a patient with hepatitis C infection. J Am Acad Dermatol. Nov 1998;39(5 Pt 2):824-6. [Medline]. 63. Carrozzo M, Carbone M, Gandolfo S, Valente G, Colombatto P, Ghisetti V. An atypical verrucous carcinoma of the tongue arising in a patient with oral lichen planus associated with hepatitis C virus infection. Oral Oncol. May 1997;33(3):220-5. [Medline]. 64. Rongioletti F, Rebora A. Worsening of lichen myxedematosus during interferon alfa-2a therapy for chronic active hepatitis C. J Am Acad Dermatol. May 1998;38(5 Pt 1):760-1. [Medline]. 65. Ferri C, Zignego AL, Bombardieri S, et al. Etiopathogenetic role of hepatitis C virus in mixed cryoglobulinemia, chronic liver diseases and lymphomas. Clin Exp Rheumatol. Nov-Dec 1995;13 Suppl 13:S135-40. [Medline]. 66. Sansonno D, Cornacchiulo V, Iacobelli AR, Di Stefano R, Lospalluti M, Dammacco F. Localization of hepatitis C virus antigens in liver and skin tissues of chronic hepatitis C virus-infected patients with mixed cryoglobulinemia. Hepatology. Feb 1995;21(2):305-12. [Medline]. 67. Kanto T, Hayashi N, Takehara T, et al. Impaired allostimulatory capacity of peripheral blood dendritic cells recovered from hepatitis C virus-infected individuals. J Immunol. May 1 1999;162(9):5584-91. [Medline]. 68. Frasca L, Del Porto P, Tuosto L, et al. Hypervariable region 1 variants act as TCR antagonists for hepatitis C virus-specific CD4+ T cells. J Immunol. Jul 15 1999;163(2):650-8. [Medline]. 69. Hartmann H, Schott P, Polzien F, et al. Cryoglobulinemia in chronic hepatitis C virus infection: prevalence, clinical manifestations, response to interferon treatment and analysis of cryoprecipitates. Z Gastroenterol. Nov 1995;33(11):643-50. [Medline]. 70. Terrier B, Sene D, Dechartres A, et al. Systemic Vasculitis in Patients with Hepatitis C Virus Infection with and without Detectable Mixed Cryoglobulinemia. J Rheumatol. Jan 2011;38(1):104-10. [Medline]. 71. Lim HW, Pereira A, Sassa S, Kim M, Zolla-Pazner S. Early-stage HIV infection and hepatitis C virus infection are associated with elevated serum porphyrin levels. J Am Acad Dermatol. Dec 1998;39(6):956-9. [Medline]. 72. Ramos-Casals M, Loustaud-Ratti V, De Vita S, et al. Sjogren syndrome associated with hepatitis C virus: a multicenter analysis of 137 cases. Medicine (Baltimore). Mar 2005;84(2):81-9. [Medline]. 73. [Guideline] U.S. Preventive Services Task Force. Screening for hepatitis C virus infection in adults: recommendation statement. Ann Intern Med. Mar 16 2004;140(6):462-4. [Medline]. [Full Text]. 74. [Guideline] British Association for Sexual Health and HIV. United Kingdom national guideline on the management of the viral hepatitides A, B, and C 2008. National Guidelines Clearinghouse. 2008. 75. Ohto H, Terazawa S, Sasaki N, et al. Transmission of hepatitis C virus from mothers to infants. The Vertical Transmission of Hepatitis C Virus Collaborative Study Group. N Engl J Med. Mar 17 1994;330(11):744-50. [Medline]. 76. Abe Y, Tanaka Y, Takenaka M, Yoshida H, Yatsuhashi H, Yano M. Leucocytoclastic vasculitis associated with mixed cryoglobulinaemia and hepatitis C virus infection. Br J Dermatol. Feb 1997;136(2):272-4. [Medline]. 77. Akiyama M, Inamoto N. Arteriovenous haemangioma in chronic liver disease: clinical and histopathological features of four cases. Br J Dermatol. Mar 2001;144(3):604-9. [Medline]. 78. Bonkovsky HL, Poh-Fitzpatrick M, Pimstone N, et al. Porphyria cutanea tarda, hepatitis C, and HFE gene mutations in North America. Hepatology. Jun 1998;27(6):1661-9. [Medline]. 79. Cacoub P, Hausfater P, Musset L, Piette JC. Mixed cryoglobulinemia in hepatitis C patients. GERMIVIC. Ann Med Interne (Paris). Feb 2000;151(1):20-9. [Medline]. 80. De Re V, De Vita S, Marzotto A, et al. Pre-malignant and malignant lymphoproliferations in an HCV-infected type II mixed cryoglobulinemic patient are sequential phases of an antigen-driven pathological process. Int J Cancer. Jul 15 2000;87(2):211-6. [Medline]. 81. Dega H, Frances C, Dupin N, et al. [Pruritus and the hepatitis C virus. The MULTIVIRC Unit]. Ann Dermatol Venereol. Jan 1998;125(1):9-12. [Medline]. 82. Eng MA, Kallemuchikkal U, Gorevic PD. Hepatitis C virus, autoimmunity and lymphoproliferation. Mt Sinai J Med. Mar 2000;67(2):120-32. [Medline]. 83. Galossi A, Guarisco R, Bellis L, Puoti C. Extrahepatic manifestations of chronic HCV infection. J Gastrointestin Liver Dis. Mar 2007;16(1):65-73. [Medline]. 84. Geissler M, Gesien A, Tokushige K, Wands JR. Enhancement of cellular and humoral immune responses to hepatitis C virus core protein using DNA-based vaccines augmented with cytokine-expressing plasmids. J Immunol. Feb 1 1997;158(3):1231-7. [Medline]. 85. Gordon SC, Patel AH, Kulesza GW, Barnes RE, Silverman AL. Lack of evidence for the heterosexual transmission of hepatitis C. Am J Gastroenterol. Dec 1992;87(12):1849-51. [Medline]. 86. Harada K, Tsuneyama K, Sudo Y, Masuda S, Nakanuma Y. Molecular identification of bacterial 16S ribosomal RNA gene in liver tissue of primary biliary cirrhosis: is Propionibacterium acnes involved in granuloma formation?. Hepatology. Mar 2001;33(3):530-6. [Medline]. 87. Kanazawa K, Yaoita H, Tsuda F, Okamoto H. Hepatitis C virus infection in patients with urticaria. J Am Acad Dermatol. Aug 1996;35(2 Pt 1):195-8. [Medline]. 88. Kontochristopoulos GJ, Aroni K, Anagnostopoulos G, Nakopoulou L, Tassopoulos NC. Erythema dyschromicum perstans and hepatitis C virus infection. Int J Dermatol. May 2001;40(5):346-8. [Medline]. 89. Laganovic M, Jelakovic B, Kuzmanic D, et al. Complete remission of cryoglobulinemic glomerulonephritis (HCV-positive) after high dose interferon therapy. Wien Klin Wochenschr. Jul 7 2000;112(13):596-600. [Medline]. 90. Longombardo G, Ferri C, Marchi S, et al. Immune response to an epitope of the NS4 protein of hepatitis C virus in HCV-related disorders. Clin Immunol Immunopathol. May 1998;87(2):124-9. [Medline]. 91. Lopes LM, Lopes EP, Silva E, et al. Prevalence of hepatitis C virus antibodies in primary glomerulonephritis in Brazil. Am J Nephrol. 1998;18(6):495-7. [Medline]. 92. Mendez P, Saeian K, Reddy KR, et al. Hepatitis C, cryoglobulinemia, and cutaneous vasculitis associated with unusual and serious manifestations. Am J Gastroenterol. Aug 2001;96(8):2489-93. [Medline]. 93. Moran MJ, Fontanellas A, Brudieux E, et al. Hepatic uroporphyrinogen decarboxylase activity in porphyria cutanea tarda patients: the influence of virus C infection. Hepatology. Feb 1998;27(2):584-9. [Medline]. 94. Polzien F, Schott P, Mihm S, Ramadori G, Hartmann H. Interferon-alpha treatment of hepatitis C virus-associated mixed cryoglobulinemia. J Hepatol. Jul 1997;27(1):63-71. [Medline]. 95. Schmidt-Mende J, Bieck E, Hugle T, et al. Determinants for membrane association of the hepatitis C virus RNA-dependent RNA polymerase. J Biol Chem. Nov 23 2001;276(47):44052-63. [Medline]. 96. Schott P, Polzien F, Muller-Issberner A, Ramadori G, Hartmann H. In vitro reactivity of cryoglobulin IgM and IgG in hepatitis C virus-associated mixed cryoglobulinemia. J Hepatol. Jan 1998;28(1):17-26. [Medline]. 97. Schott P, Pott C, Ramadori G, Hartmann H. Hepatitis C virus infection-associated non-cryoglobulinaemic monoclonal IgMkappa gammopathy responsive to interferon-alpha treatment. J Hepatol. Aug 1998;29(2):310-5. [Medline]. 98. Thomas DL, Astemborski J, Rai RM, et al. The natural history of hepatitis C virus infection: host, viral, and environmental factors. JAMA. Jul 26 2000;284(4):450-6. [Medline]. 99. Toth CM, Pascual M, Chung RT, et al. Hepatitis C virus-associated fibrosing cholestatic hepatitis after renal transplantation: response to interferon-alpha therapy. Transplantation. Nov 15 1998;66(9):1254-8. [Medline].
|
|
|
|