|
Chronic liver disease and
skeletal health (hepatic
osteodystrophy)
T Bandgar,
V Shivane,
A Lila,
N Shah
Department of Endocrinology,
Seth G.S. Medical College
and K.E.M. Hospital, Mumbai,
Maharashtra, India
| Date
of Web Publication |
14-Jun-2012 |
Correspondence Address:
T Bandgar
Department of Endocrinology,
Seth G.S. Medical College
and K.E.M. Hospital, Mumbai,
Maharashtra
India

DOI:
10.4103/0022-3859.97170
How to cite this
article:
Bandgar T, Shivane
V, Lila A, Shah N.
Chronic liver
disease and skeletal
health (hepatic
osteodystrophy). J
Postgrad Med
2012;58:103-6 |
Metabolic bone disease is a
common complication of
chronic liver disease (CLD),
ranging from cholestatic
disorders to alcoholic,
autoimmune and post-viral
cirrhosis.
[1]
Often known as hepatic
osteodystrophy (HO), this is
of two types: 1.
osteoporosis that is similar
to post-menopausal and
aging-related bone loss;
this type is more frequently
seen and trabecular (cancellous)
bone is more severely
affected than cortical bone;
2. osteomalacia that is
found in cases of advanced
liver disease, in the
presence of severe
cholestasis (clinically
manifested as jaundice) and
malabsorption. In CLD,
analyses have generally been
performed not only in
cirrhotics with a broad
range of disease severity
but also in pre-cirrhotic
patients. The etiology of HO
is poorly understood and is
thought to vary according to
the type, severity and
progression of the liver
disease, along with a
multitude of other
contributing factors,
including the ethnicity of
the population studied. It
can result in spontaneous
low-trauma fractures that
significantly impact
survival and quality of
life, through pain,
deformity and immobility.
With orthotopic liver
transplantation (OLT)
steadily taking the center
stage in the treatment of
end-stage cirrhosis and
offering long-term survival,
bone disease has snowballed
into one of the major
determinants of survival and
quality of life in this
cohort.
[1] Several
cross-sectional and
longitudinal studies have
shown, despite considerable
heterogeneity in case
selection and methodology,
that individuals with CLD
have a pronounced loss of
bone mineral density (BMD)
(osteoporosis prevalence of
20-50%) and a moderately
increased rate of
osteoporotic fractures
(5-20%).
[2] Sachdev et
al.
[3] and George
et al.
[4] have reported
low BMD in 64% and 68% of
Asian Indian patients with
CLD respectively.
|
:: Etiopathogenesis
and Mechanism of
Osteoporosis in CLD |
|
 |
The major controversy
regarding the mechanism of
osteoporosis in CLD is
whether it is because of
less bone formation or more
bone resorption. In
low-turnover osteoporosis
(80%), bone remodeling unit
(BMU) activity is severely
affected (parenchymal liver
disease), whereas in
high-turnover osteoporosis
(20%), BMU activity is
increased (cholestatic liver
diseases).
[5] In our study
of Asian Indian patients
with non-cholestatic
etiology of liver diseases,
the mechanism of HO has been
shown to be due to decreased
bone formation with
increased bone resorption.
[4]
Loss of bone mass is quite a
common finding in chronic
hepatic dysfunction.
Potential inciting factors
that either directly or
indirectly alter bone mass
include insulin growth
factor-I (IGF-I) deficiency,
hyperbilirubinemia,
hypogonadism (estrogen and
testosterone deficiency),
alcoholism, excess tissue
iron deposition, subnormal
vitamin D levels, vitamin D
receptor genotype and
osteoprotegerin (OPG) and
receptor activator of
nuclearfactor Kb ligand (RANKL)
interactions. Furthermore,
immunosupressants and
antiviral agents such as
interferon and ribavirin may
affect bone metabolism.
[5] Cholestatic
disease per se does
not differ significantly
from non-cholestatic
disorders in terms of
osteoporosis and fracture
risk.
[2]
|
:: Insulin Growth
Factor-I |
|
 |
The source is the liver and
osteoblast, which is
important for the
development and maintenance
of bone mass. Earlier
studies have shown that the
severity of CLD from chronic
hepatitis to cirrhosis is
associated with a
progressive increase of
growth hormone resistance
and with low IGF-I serum
levels.
[6]
|
:: Bilirubin |
|
 |
Increased levels found in
CLD patients results in
decreased IGF-1 generation,
and has an inhibitory effect
on the osteoblast. However,
Smith et al.
concluded that
hyperbilirubinemia is not a
major contributing factor
for low bone mass in
patients with CLD.
[7]
|
:: Hypogonadism |
|
 |
It is an established risk
factor for osteoporosis, and
CLD accelerates the
development of hypogonadism
due to altered hypothalmo-pituitary
function with reduced
release of gonadotrophins,
and primary gonadal failure.
|
:: Vitamin D |
|
 |
In CLD patients, a subnormal
serum concentration of
vitamin D is not a
consequence of reduced
hepatic hydroxylation, but
is due to malabsorption,
increased urinary excretion
and reduced enterohepatic
circulation of Vitamin D.
Given the ubiquitous
prevalence of Vitamin D
deficiency in the Asian
Indian population in all the
age groups, this may prove
to be an important
contributory factor in this
part of the subcontinent.
Subsequent hepatic
25-hydroxylation of vitamin
D3 has not been studied in
humans but, in cirrhotic
rats, this process is not
impaired.
[8],[5]
Reduced tissue sensitivity
to circulating Vitamin D due
to altered Vitamin D
receptor genotypes may also
play a role in the
development of HO. Vitamin D
receptor allelic
polymorphisms, designated
B/b, A/a and T/t alleles,
correlate with BMD. The risk
of developing a vertebral
fracture increased two- to
three-fold with the presence
of a T/t allele.
[5]
|
:: Vitamin K |
|
 |
In CLD, deficiency of
Vitamin K is also seen,
especially in cholestatic
liver disease. Vitamin K is
required for the formation
of osteocalcin and
osteonectin. Supplementation
of Vitamin K is associated
with improvements in BMD.
Furthermore, Vitamin K2
inhibits expression of
ligand (RANKL), tartrate-resistant
acid phosphatase (TRAP)
activity, mononuclear cell
formation and also induces
osteoclast apoptosis in
vitro.[9]
|
:: Osteoprotegerin
and Receptor
Activator of
Nuclearfactor kb
Ligand |
|
 |
OPG (tumor necrosis factor
receptor super family) is
produced by the liver and it
inhibits osteoclast
differentiation, whereas
RANKL plays a role in the
differentiation and
activation of osteoclasts by
binding to its high-affinity
receptor (RANK) located on
the surface of the
osteoclasts. The role of OPG
in hepatic osteodystrophy is
not yet clear. Studies have
shown that circulating OPG
is increased and soluble
RANKL is decreased,
regardless of osteoporosis,
contrary to the expectation
in CLD.
[10] Probably,
there is a qualitative
change in the OPG/RANKL
system that contributes to
the low bone mass in CLD
patients.
|
:: Medications |
|
 |
Corticosteroid forms the
therapeutic component for
autoimmune hepatitis and for
immunosuppression after
liver transplantation.
Prolonged steroid therapy
results in clinically
significant bone loss with
an increase in fracture risk
by greater than two-fold.
[11] Steroids
exert a direct effect on the
bone cells by increasing the
osteoclastic activity by
increasing IL1 and IL6 and
decreasing differentiation,
recruitment and life span of
osteoblast. Calcineurin
inhibitors
[12] are used in
conjunction with
corticosteroids; therefore,
the independent effect of
these agents on bone
metabolism in humans is
difficult to ascertain.
Additional medications used
in the treatment of advanced
liver disease, such as
diuretics, anticoagulants
and chemotherapy, also have
a deleterious effect on the
bone.
|
:: Alcohol |
|
 |
Osteoporosis is frequently
observed in the alcoholic
patient. Ethanol decreases
bone formation in a
dose-dependent fashion,
mainly through a direct
toxic effect on osteoblast
function.
[2] It also
alters, both directly and
indirectly, bone mineral
metabolism including PTH,
Vitamin D, testosterone,
IGF-1, cytokines (raised TNF
and IL-6) and cortisol
levels.
|
:: Iron |
|
 |
An increased iron burden has
been associated with
impaired osteoblast
activity. Excess pituitary
iron deposition (genetic
hemachromatosis) may also
contribute to the
development of hypogonadism
independent of the cirrhotic
process.
[2]
Contributing factors for low
bone mass in our study on
the Asian Indian population
with non cholestatic liver
cirrhosis were inadequate
sunlight exposure, reduced
physical activity, low lean
body mass, Vitamin D
deficiency and hypogonadism,
along with IGF-1 deficiency
and low estrogen in men.
[4] The presence
of most risk factors in low
and normal BMD groups
indicated that all Asian
Indian patients with
cirrhosis are vulnerable
and, unless prevented, will
develop the disease
|
:: Clinical
Presentation |
|
 |
Clinically, these patients
present with bone pains,
backache, loss of height,
fragility fractures and
kyphosis/scoliosis.
|
:: Diagnosis |
|
 |
Various biochemical tests
may be useful to ascertain
calcium metabolism and
gonadal hormone status:
serum calcium, phosphate,
thyroid function tests,
intact parathyroid hormone,
25-hydroxyvitamin D,
bioavailable testosterone
(men), serum estradiol and
follicular stimulating
hormone, luteinizing
hormone. Other tests include
X-ray, dual energy X-ray
absorptiometry (DXA) scan,
quantitative computerized
tomography and biochemical
markers of bone disease.
|
:: Dual Energy X-ray
Absorptiometry |
|
 |
Indications
Chronic cholestasis, alcohol
abuse, post-menopausal women
with additional risk factors
for osteoporosis, male
hypogonadism, long-term
corticosteroid therapy (more
than 3 months), any patient
with a fragility fracture,
low body mass index and
evaluation for
transplantation.
Further monitoring with
DXA
(1) Patients with normal BMD:
2-3 yearly; (2) high risk
characters, viz. in
cholestatic patients with
more than one risk factor
for osteoporosis, and in
those recently initiating
high-dose corticosteroid
therapies: 1 yearly.
|
:: Biochemical
Markers |
|
 |
Biochemical markers of bone
disease, viz. bone formation
(procollagen propeptides of
type 1 collagen, osteocalcin
and bone isoenzyme of
alkaline phosphatase), while
bone resorption (urinary
excretion of
deoxypyridinoline,
pyridinoline and Type 1
collagen cross-linked N-telopeptide)
has not been studied in
patients with CLD. Hence, it
cannot be recommended.
|
:: Management |
|
 |
The prevention of fragility
fractures, and not the
improvement of BMD, is the
ultimate clinical goal for
patients with osteoporosis.
It is vital to optimize
other factors that help
reduce the risk of falls and
fractures. The clinical
approach is depicted in
[Figure 1].
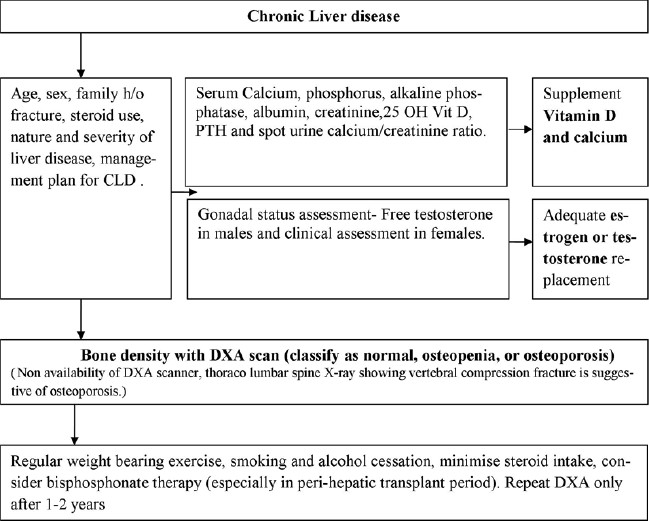 |
Figure 1: Approach
to the clinical
management of
osteoporosis in
patients with
chronic liver
disease
Click here to
view |
With the advent of OLT in
the management of CLD, two
stages of intervention for
improving bone mass may be
suggested: pre- and post-OLT.
Pre-orthotopic liver
transplantation
Low bone turnover
[12],[13],[14]
is present before the
procedure. It has been shown
that low BMD and the
presence of vertebral
fractures before OLT are
strong predictors of
post-transplant fragility
fractures.
[2] Therefore,
efforts to optimize and
preserve lifetime bone mass
should be initiated very
early in all patients with
progressive CLD,
irrespective of whether
transplantation is on the
horizon or not, and
continued through all the
stages.
The following general
measures are a must:
- Moderate physical
exercise
- Smoking and alcohol
cessation
- Maintaining good
nutritional state
calcium (1000-1500 mg/d)
and Vitamin D (800-1000
IU/d) and ensure
adequate blood levels of
25-OH Vitamin D (20-30
ng/mL)
- Treating
hypogonadism if present
and if there are no
contraindications
- Initiating
antiresorptive treatment
according to BMD,
fractures and other risk
factors. Vitamin D
deficiency should be
corrected before giving
bisphosphonates after
OLT to prevent
hypocalcemia.
Post-orthotopic liver
transplantation
Bone loss and fracture rates
after OLT are highest in the
first 6-12 months. Spine BMD
declined by 2-24% during the
first year in earlier
studies,
[12] followed by
an improvement of BMD 12
months post-transplantation.
Fracture rates range from 24
to 65%, and the ribs and
vertebrae are the most
common sites.
[12] Women with
primary biliary cirrhosis
and the most severe
pre-existing bone disease
appear to be at greatest
risk.
After OLT, bone turnover is
increased, but the cause of
rapid bone loss immediately
after OLT is not completely
understood. Several factors,
including post-operative
immobility and high-dose
glucocorticoid treatment,
are likely to play a role.
Prevention and treatment
Both oral and iv
bisphosphonates are
effective in reducing post-OLT
bone loss. A 12-month
randomized study of 30 mg iv
pamidronate every 3 months
showed that pamidronate
increased spinal BMD but did
not prevent femoral neck
bone loss.
[15] A randomized
trial of iv bandronate in
OLT recipients prevented
bone loss at 1 year. A
randomized, double-blind
trial of adults having liver
transplantation showed that
infusions of 4 mg
zolendronic acid within 7
days of liver
transplantation and again at
1, 3, 6 and 9 months after
OLT
[16] reduced bone
loss by 3.8-4.7% at the LS,
femoral neck and total hip
compared with patients
receiving saline infusions.
At 12 months after
transplantation, the
differences only remained
significant at the total
hip. One study used
historical controls to
examine the effects of
alendronate in addition to
calcium and calcitriol 0.5
mcg daily after LT.
Increases in spinal, femoral
neck and total hip BMD at 12
months were higher than in
historical controls.
[17]
|
:: Children |
|
 |
Management of low bone mass
in children is similar to
that in adults, where the
role of prevention is most
important with Vitamin
D/calcium, physical
activity, esp vibrating
platform to stimulate muscle
activity and, consequently,
bone strength. In the
management of children who
have sustained osteoporotic
fractures, the treatment for
which currently there is the
most evidence of benefit is
bisphosphonates.
[14],[18]
|
:: Controversies and
Areas of Uncertainty |
|
 |
Older agents, viz. hormone
replacement therapy,
calcitonin and strontium
ranelate have fallen out of
favor in the management of
osteoporosis either due to
their side-effect profile or
inefficacy.
- Newer agents, viz.
PTH (1-34) or
teriparatide (anabolic
agents) and denosumab:
human antibodies to
RANKL and cathepsin K
inhibitors (anticatabolic
agents) need to undergo
clinical studies to test
their efficacy in CLD
patients
- Regarding
bisphosphonates, which
remains the mainstay of
treatment for
transplantation bone
disease, several issues
remain unanswered
- optimal route
- frequency
- duration of
therapy
- dose in renal
failure
- long-term data
with fracture
endpoints with
intervention.
To summarize, the
association of CLD and
decreased BMD is well
established, but
hepatologists may be
distracted from this fact by
a patient's relatively young
age and a list of other
medical conditions to deal
with. Increased vigilance to
diagnose and taking measures
to prevent HO is the need of
the hour.
|
:: References |
|
 |
|
1. |
Sanchez AJ,
Aranda-Michel J.
Liver disease and
osteoporosis. Nutr
Clin Pract 2006;21:
273-8.

[PUBMED]
[FULLTEXT]
|
|
2. |
Leslie WD,
Bernstein CN, Leboff
MS. American
Gastroenterological
Association Clinical
Practice Committee.
AGA technical report
on osteoporosis in
hepatic disorders.
Gastroenterology
2003;125:941-66.

|
|
3. |
3 Sachdev S,
Bhasin RC, Kumari
CK, Reys M. A study
of metabolic bone
disorder in
cirrhosis liver. J
Assoc Physicians
India 1976;24: 5-11.

|
|
4. |
George J, Ganesh
HK, Acharya S,
Bandgar TR, Shivane
VK, Karvat A, et
al. Bone mineral
density and
disorders of mineral
metabolism in
chronic liver
disease. World J
Gastroenterol
2009;15:3516-22.

|
|
5. |
Goel V, Kar P.
Hepatic
Osteodystrophy. Trop
Gastroenterol
2010;31:82-6.

[PUBMED]
|
|
6. |
Assy N,
Pruzansky Y, Gaitini
D, Orr ZS, Hochberg
Z, Baruch Y. Growth
hormone-stimulated
IGF-1 generation in
cirrhosis reflects
hepatocellular
dysfunction. J
Hepatol
2008;49:34-42.

|
|
7. |
Smith DL, Shire
NJ, Watts NB,
Schmitter T, Szabo
G, Zucker SD.
Hyperbilirubinemia
is not a major
contributing factor
to altered bone
mineral density in
patients with
chronic liver
disease. J Clin
Densitom
2006;9:105-13.

[PUBMED]
[FULLTEXT]
|
|
8. |
Collier J. Bone
disorders in chronic
liver disease.
Hepatology
2007;46:1271-8.

[PUBMED]
[FULLTEXT]
|
|
9. |
Cockayne S,
Adamson J,
Lanham-new S,
Shearer MJ, Gilbody
S, Torgerson DJ.
Vitamin K and
prevention of
fractures:
Systematic review
and meta-analysis of
randomized
controlled trials.
Arch Intern Med
2006;166:1256-61.

[PUBMED]
[FULLTEXT]
|
|
10. |
Elsedfy HH.
Hepatic
osteodystrophy.
Egypt Liver J
2011;1:8-10.

|
|
11. |
Canalis E,
Mazziotti G,
Giustina A,
Bilezikian JP.
Glucortiocid-induced
osteoporosis:
Pathophysiology and
therapy. Osteoporos
Int 2007;18:1319-28.

[PUBMED]
[FULLTEXT]
|
|
12. |
Karges W,
Trautwein C. Liver
transplantation and
osteoporosis:
Securing "Bone-fied"
success. Liver
Transpl
2006;12:1322-3.

[PUBMED]
[FULLTEXT]
|
|
13. |
Guichelaar MM,
Kendall R, Malinchoc
M, Hay JE. Bone
mineral density
before and after
liver
transplantation:
Long-term follow up
and predictive
factors. Liver
Transpl
2006;12:1390-402.

[PUBMED]
[FULLTEXT]
|
|
14. |
Ebeling P.
Approach to the
patient with
transplantation-related
bone loss. J Clin
Endocrinol Metab
2009;94:1483-90.

|
|
15. |
Monegal A,
Guanabens N, Suarez
MJ, Suarez F,
Clemente G,
Garcia-Gonzalez M,
et al.
Pamidronate in the
prevention of bone
loss after liver
transplantation: A
randomized
controlled trial.
Transpl Int
2009;22:198-206.

|
|
16. |
Misof BM,
Bodingbauer M,
Roschger P, Wekerle
T, Pakrah B, Haas M,
et al.
Short-term effects
of highdose
zoledronic acid
treatment on bone
mineralization
density distribution
after orthotopic
liver
transplantation.
Calcif Tissue Int
2008;83:167-75.

[PUBMED]
[FULLTEXT]
|
|
17. |
Atamaz F,
Hepguler S, Karasu
Z, Kilic M, Tokat Y.
The prevention of
bone fractures after
liver
transplantation:
Experience with
alendronate
treatment.
Transplant Proc
2006;38:1448-52.

|
|
18. |
De Albuquerque
Taveira AT,
Fernandes MI, Galvao
LC, Sawamura R, De
Mello Vieira E,
et al.
Impairment of bone
mass development in
children with
chronic cholestatic
liver disease. Clin
Endocrinol (Oxf)
2007;66:518-23.
 |
|