|
Hepatitis C
Virus Transmission at an
Outpatient Hemodialysis Unit
--- New York, 2001--2008
In July
2008, the New York State
Department of Health (NYSDOH)
received reports of three
hemodialysis patients
seroconverting from
anti-hepatitis C virus (HCV)
negative to anti-HCV
positive in a New York City
hemodialysis unit during the
preceding 6 months. NYSDOH
conducted patient interviews
and made multiple visits to
the hemodialysis unit to
observe hemodialysis
treatments, assess infection
control practices, evaluate
HCV surveillance activities,
review medical records, and
conduct interviews with
staff members. This report
summarizes the results of
that investigation, which
found that six additional
patients had HCV
seroconversion during
2001--2008 and that the
hemodialysis unit had
numerous deficiencies in
infection control policies,
procedures, and training. Of
the total of nine
seroconversions, the sources
for four HCV infections were
identified phylogenetically
and epidemiologically as
four other patients in the
unit. The unit's policy for
routine patient testing for
HCV infection was not in
accordance with CDC
recommendations, and the few
recommendations followed
were not implemented
consistently. Hemodialysis
units should routinely
assess compliance to ensure
complete and timely
adherence with CDC
recommendations to reduce
the risk for HCV
transmission in this
setting.
The
hemodialysis unit was a
large, for-profit,
outpatient facility treating
70--100 patients daily at 30
dialysis stations. On May
24, 2008, the New York City
Department of Health and
Mental Hygiene informed
NYSDOH of a confirmed HCV
seroconversion in one
patient receiving chronic
hemodialysis treatment at
the unit. On July 1, the
unit reported two additional
HCV seroconversions directly
to NYSDOH. Interviews
conducted by NYSDOH with the
three patients who
seroconverted revealed no
other common health-care
exposures or behavioral risk
factors. In addition, none
of the three had been
informed by the hemodialysis
unit of their HCV
infections. Initial site
visit findings by NYSDOH
documented poor infection
control practices and
oversight. Specific
recommendations addressing
deficiencies were provided
to the unit's administrative
staff members at the initial
site visit and throughout
the investigation. An
epidemiologic investigation
subsequently was undertaken
to identify additional
patients with HCV infection,
assess infection control
practices, and make
recommendations to prevent
ongoing transmission.
Epidemiologic Investigation
The
epidemiologic study
population consisted of all
162 patients who were
receiving hemodialysis at
the unit as of July 1, 2008.
For all patients,
HCV-related test results
reported through the unit's
central electronic
laboratory system and the
NYSDOH Electronic Clinical
Laboratory Reporting System
were reviewed, and patients
were matched against New
York state and New York City
hepatitis surveillance
registries. All current
patients were offered
anti-HCV testing. Because
hemodialysis unit staff
members were not considered
likely sources of HCV
transmission in this
investigation, staff members
were not tested.
Patients
were considered HCV positive
if their serum was 1)
determined positive by
enzyme immunoassay (EIA)
testing with a
signal-to-cutoff ratio
consistent with CDC
recommendations for a
confirmed anti-HCV positive
test or 2) determined
positive by EIA followed by
recombinant immunoblot assay
or nucleic acid testing for
HCV RNA (1).
A chronic case of HCV was
defined as a case in a
patient who was HCV positive
before or upon admission to
the hemodialysis unit. An
incident case was defined as
a case in a patient who was
HCV negative upon admission
to the hemodialysis unit but
who subsequently was
confirmed HCV positive. Unit
medical records for all
HCV-positive patients were
reviewed, and serum from
available patients was
submitted to NYSDOH's
Wadsworth Center laboratory
for HCV sequencing and
phylogenetic analysis.
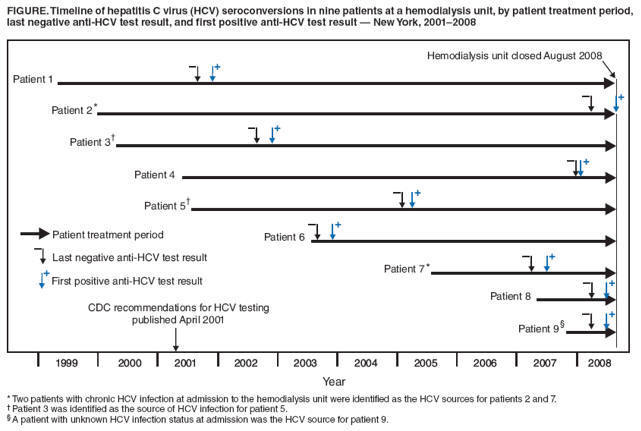
Of the 162
patients, HCV infection
status at hemodialysis unit
admission could be
documented through medical
records and previous test
results for 110 (68%).
Twenty (18%) of the 110 had
chronic HCV infection at
admission. Ninety (82%) were
anti-HCV negative at
admission, of whom nine
(10%) were determined to
have acquired incident HCV
infection, seroconverting to
anti-HCV positive during
2001--2008 (Figure).
Among the
162 patients, a total of 45
(28%) had at least one
anti-HCV positive EIA test
result, either at admission
or during their hemodialysis
treatment period. Serum was
collected and tested from 35
of these patients, of whom
26 had sufficient virus for
sequencing and subtyping of
NS5b region: eight of the
nine patients with incident
infection, 12 patients with
chronic infection upon
admission, and six patients
whose HCV admission status
was unknown. An HCV source
patient was defined as an
HCV-positive patient 1) with
a >95% sequence
identity match in the NS5b
region of the HCV genome
with a patient with incident
infection and 2) who had
received hemodialysis
treatment on recurring days
at the same time as the
patient with incident
infection, during the
seroconverting patient's
exposure period (i.e., from
6 months before the
patient's last negative
anti-HCV test through 2
weeks before the first
positive anti-HCV test). The
joint phylogenetic-epidemiologic
analysis identified four
different patients as the
sources of HCV infection in
four patients who
seroconverted during
2005--2008 (all sequence
identity matches
between source and incident
patients were >98%).
Of the four source patients,
one was among the nine with
incident infection, two were
among those with chronic HCV
infection at admission, and
one had unknown HCV
infection status at
admission. All four patients
with incident infection and
their respective source
patients had dozens of
treatment days in common
(range: 59--121 days). Two
of the four patients with
incident infection had at
least one treatment on the
same dialysis machines as
their HCV source patients;
however, no record existed
of the other two with
incident infection having
been treated during their
incubation periods on the
same machines as their
source patients.
HCV source
patients could not be
determined for five of the
patients with incident
infection because no
sequence identity match was
identified. None of the five
had known HCV risk factors
(e.g., occupational
exposure, injection-drug
use, high-risk sexual
behaviors, or exposure to
known HCV-positive persons).
Two of the five reported no
health-care exposures
outside of the hemodialysis
unit during their exposure
periods; the other three
reported respectively 1) one
emergency department visit,
2) one hospitalization, and
3) one emergency department
visit and two hospital
admissions. Epidemiologic
analysis is continuing in an
effort to define narrower
exposure periods and
determine the mechanism or
mechanisms of HCV
transmission at this
facility.
Site
Investigation
During the
site investigation, NYSDOH
documented inadequate HCV
infection surveillance and
patient follow-up (2).
Numerous deficiencies in
standard infection control
practices also were
identified (2).
The hemodialysis unit did
not obtain confirmatory
testing for anti-HCV
positive results, inform
patients of their change in
HCV infection status, report
HCV seroconversions to the
local health department, or
provide patients with
medical evaluation related
to HCV infection. Contrary
to CDC recommendations (2),
monthly alanine
aminotransferase (ALT)
levels were not obtained
from >90% of HCV-susceptible
patients, and anti-HCV
testing, although conducted
on most patients, was
performed at intervals
ranging from once per month
to once per 2 years rather
than semiannually.
Inadequate
cleaning and disinfection
practices were observed
during site visits in July
and August 2008. A single
bleach-soaked gauze pad was
used to clean a patient's
entire dialysis station,
including dialysis machine
surfaces and ancillary
patient equipment (e.g.,
blood pressure cuff and
shared computer monitor and
keyboard). The bleach
solution was prepared and
stored improperly, and staff
members did not allow
sufficient contact time
between surfaces and bleach.
Visible blood remained on
dialysis chairs, dialysis
machine surfaces, and the
surrounding floor between
patient treatments.
Moreover, direct care staff
members failed to don gloves
with every patient
encounter, change gloves
between patients, or perform
hand hygiene after contact
with patients and soiled
surfaces. Supervisory staff
members failed to address
these breaches. Many of the
direct care staff members
were unaware of the
hemodialysis unit's written
infection control policies,
including those pertaining
to cleaning and
disinfection. Investigators
also noted the lack of a
separate clean area for
medication storage and
preparation and short
turnover periods between
patient treatments.
On August
14, 2008, after evidence of
ongoing infection control
deficiencies and despite
efforts at remediation,
NYSDOH directed the
hemodialysis unit to
transfer all patients
immediately to other
facilities; all patients
were transferred the next
day. The hemodialysis unit
subsequently surrendered its
operating certificate and
paid a $300,000 civil
penalty to the state; the
unit has not reopened. Based
on evidence of HCV
transmission since 2005, all
patients who had received
one or more treatments at
the hemodialysis unit since
January 23, 2004 (the date
of the last facility survey
in which no infection
control deficiencies were
observed) were notified by
mail of the investigation
and advised to be tested for
HCV and other bloodborne
pathogens (i.e., hepatitis B
virus and human
immunodeficiency virus).
Notification letters were
mailed on September 15,
2008, to a total of 657
patients from 37 states and
two territories. As of
January 11, 2009, no
additional HCV
seroconversions had been
reported from health
departments in New York, 13
other states, and one
territory, accounting for
90% of the patients who were
notified.
Reported
by: R Hallack, G
Johnson, MS, E Clement, MSN,
M Parker, PhD, J Schaffzin,
MD, PhD, B Wallace, MD, P
Smith, MD, New York State
Dept of Health; ND Thompson,
PhD, Div of Viral Hepatitis,
National Center for
HIV/AIDS, Viral Hepatitis,
STD, and TB Prevention; PR
Patel, MD, JF Perz, DrPH,
Div of Healthcare Quality
Promotion, National Center
for Preparedness, Detection,
and Control of Infectious
Diseases; J Magri, MD,
Career Development Div,
Office of Workforce and
Career Development; JL
Jaeger, MD, EIS Officer,
CDC.
Editorial
Note:
An estimated
3.2 million persons have
chronic HCV infection, the
most common chronic
bloodborne infection in the
United States (3).
The prevalence of anti-HCV
is estimated at 8% among
chronic hemodialysis
patients (4),
compared with 1.6% in the
U.S. population overall (3).
HCV infection increases the
risk for death among
patients receiving chronic
hemodialysis treatment and
those undergoing renal
transplantation (5).
Many persons infected with
HCV remain asymptomatic,
although progression of
underlying liver disease
occurs in approximately 80%
(6).
Chronic HCV infection is the
leading indication for liver
transplantation in the
United States (7).
CDC
recommendations for
preventing HCV transmission
in hemodialysis units were
published in 2001 (Box)
(2).
Despite these
recommendations, several
hemodialysis-related HCV
outbreaks have occurred in
recent years; all involved
breaches in infection
control, and most were
identified as a result of
routine HCV screening (8).
CDC recommends initial
anti-HCV screening upon
admission to the unit for
all chronic hemodialysis
patients. For
HCV-susceptible patients,
monthly ALT should be
performed; anti-HCV
screening should be obtained
semiannually thereafter and
in response to unexplained
elevations in ALT, to
facilitate early detection
of transmission and
implementation of control
measures (2).
Routine HCV screening of
hemodialysis patients also
is recommended by the
National Kidney Foundation (9).
However, dialysis providers
are not reimbursed by
Medicare for anti-HCV
screening, and screening is
not required by the Centers
for Medicare and Medicaid
Services (10). In the
2008 Medicare conditions for
coverage for end stage renal
disease facilities (10),
CDC recommendations for
preventing transmission of
infections in hemodialysis
units (2)
were incorporated by
reference, with the
exception of screening for
hepatitis C. The referenced
recommendations have the
authority of regulation.
This
investigation documented
four cases of
patient-to-patient
transmission of HCV
infection and identified
five additional patients who
might have acquired HCV
infection while receiving
treatment at the
hemodialysis unit. Multiple
possible mechanisms of HCV
transmission were
identified, including
contaminated health-care
worker hands and treatment
surfaces. Contact
transmission in the setting
of extensive environmental
contamination is a common
mechanism for transmission
of bloodborne pathogens in
hemodialysis units (2).
Because this investigation
was restricted to patients
undergoing treatment as of
July 31, 2008, the actual
number of incident cases at
the hemodialysis unit might
have been larger.
This
outbreak highlights the need
for hemodialysis units to
adhere to recommendations
for infection control and
comprehensive HCV
surveillance, including
routine anti-HCV screening,
confirmatory testing of
anti-HCV seroconversions,
assessment of the adequacy
of infection control
practices in the setting of
documented HCV
seroconversion, and prompt
reporting to the local
health department as
required by reportable
disease laws or regulations.
Had the hemodialysis unit in
this report complied with
these practices, HCV
transmission might have been
identified earlier, and
control measures (e.g.,
reviewing infection control
practices to identify
potential mechanisms of
transmission, ensuring
adherence to unit infection
control policies, and
retraining direct care staff
members) could have been
implemented to interrupt
further HCV transmission.
Because many patients with
HCV infection are
asymptomatic, routine
screening is essential to
detect transmission within
hemodialysis facilities and
ensure that appropriate
precautions are being
followed consistently.
Acknowledgments
This report
is based, in part, on
contributions by E Rocchio,
MA, K Southwick, MD, N
Sureshbabu, and T Kwechin,
New York State Dept of
Health; and K Bornschlegel,
MPH, New York City Dept of
Health and Mental Hygiene.
References
-
CDC. Guidelines for
laboratory testing and
result reporting of
antibody to hepatitis C
virus. MMWR 2003;52(No.
RR-3).
-
CDC. Recommendations for
preventing transmission
of infections among
chronic hemodialysis
patients. MMWR
2001;50(No. RR-5).
-
Armstrong GL, Wasley A,
Simard EP, McQuillan GM,
Kuhnert WL, Alter MJ.
The prevalence of
hepatitis C virus
infection in the United
States, 1999 through
2002. Ann Intern Med
2006;144:705--14.
-
Finelli
L, Miller JT, Tokars JI,
Alter MJ, Arduino MJ.
National surveillance of
dialysis-associated
diseases in the United
States, 2002. Seminars
in Dialysis
2005;18:52--61.
-
Kamar N,
Ribes D, Izopet J,
Rostaing L. Treatment of
hepatitis C virus
infection (HCV) after
renal transplantation:
implications for
HCV-positive dialysis
patients awaiting a
kidney transplant.
Transplantation
2006;82:853--6.
-
CDC. Surveillance for
acute viral
hepatitis---United
States, 2006. MMWR
2008;57(No. SS-2).
-
Sharara
AI, Hunt CM, Hamilton
JD. Hepatitis C. Ann
Intern Med
1996;125:658--68.
-
Thompson
ND, Perz JF, Moorman AC,
Holmberg SD. Nonhospital
health care--associated
hepatitis B and C virus
transmission: United
States, 1998--2008. Ann
Intern Med
2009;150:33--9.
-
Gordon
CE, Balk EM, Becker BN,
et al. KDOQI US
commentary on the KDIGO
clinical practice
guideline for the
prevention, diagnosis,
evaluation, and
treatment of hepatitis C
in CKD. Am J Kidney Dis
2008;52:811--25.
-
Centers
for Medicare and
Medicaid Services,
Center for Medicaid and
State Operations/Survey
and Certification Group.
End Stage Renal Disease
(ESRD) Program:
interpretive guidance
version 1.1. Baltimore,
MD: Centers for Medicare
and Medicaid Services;
2008. Available at
http://www.cms.hhs.gov/eog/downloads/eo%200526.pdf.
Figure
Return to
top.
Box

Return to
top.
Use of
trade names and
commercial sources
is for
identification only
and does not imply
endorsement by the
U.S. Department of
Health and Human
Services.
References to
non-CDC sites on the
Internet are
provided as a
service to MMWR
readers and do not
constitute or imply
endorsement of these
organizations or
their programs by
CDC or the U.S.
Department of Health
and Human Services.
CDC is not
responsible for the
content of pages
found at these
sites. URL addresses
listed in MMWR
were current as of
the date of
publication.
|
All MMWR
HTML versions of articles
are electronic conversions
from typeset documents. This
conversion might result in
character translation or
format errors in the HTML
version. Users are referred
to the electronic PDF
version (http://www.cdc.gov/mmwr)
and/or the original MMWR
paper copy for printable
versions of official text,
figures, and tables. An
original paper copy of this
issue can be obtained from
the Superintendent of
Documents, U.S. Government
Printing Office (GPO),
Washington, DC 20402-9371;
telephone: (202) 512-1800.
Contact GPO for current
prices.
**Questions or messages
regarding errors in
formatting should be
addressed to
mmwrq@cdc.gov.
Date last reviewed: 3/5/2009
td>
|