|
|
||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||
|
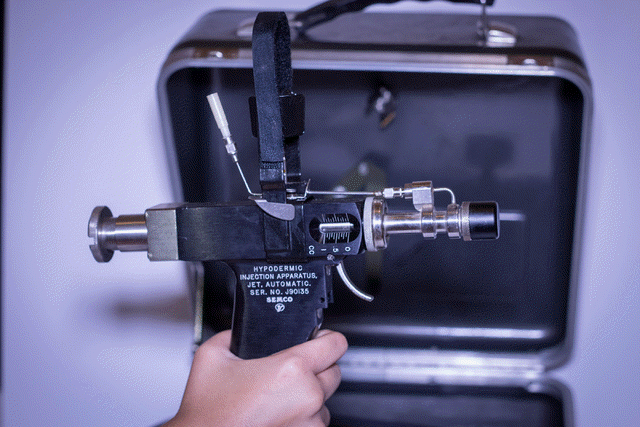
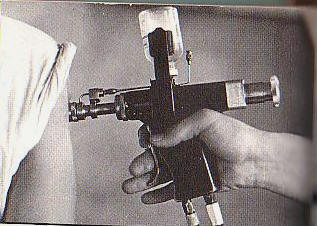
Military discontinues the use of jet
guns for mass immunization of military troops
U.S. Department of Defense (DoD) needle-free injection policy chronology (1997-11-20) Ped-O-Jet® manufacturer
(Keystone Industries, Cherry Hill, NJ) notifies
Defense Supply Center Philadelphia (DSCP)
(Defense Logistics Agency) of intent to withdraw as device supplier
over liability concern for bloodborne disease transmission from
multiple-use-nozzle design. (1997-12-05) DoD
Medical Materiel Quality Control Program (MMQCP)
issues
withdrawal of automatic jet hypodermic injection
units (MMQC-97-1169). (1997-12-07)
DSCP issues Medical Products Quality
Control System (MPQCS) device alert (DSCP 970147) as "cautionary
measure", while noting the absence of bloodborne disease transmission
case reports over 35 years of military use (followup
MMQC-98-1019 dated 1998-Jan-30). (1998-01-09) Armed Forces
Epidemiological Board (AFEB) concurs with withdrawal of Ped-O-Jet® for
"routine immunization", but availability for "public health
emergency". AFEB recommends use of "newer technology" devices with
disposable parts for skin contact. (1998-04-20)
Navy Bureau of Medicine and Surgery
updates via
BUMED notice 6230 its Immunization
Requirements And Recommendations document (3.6Mb .pdf)
prohibiting jet injector use until otherwise directed. (1998-04-28) AFEB recommends DoD
formulate new needle-free injector specifications and support device
research and development. (1998-07-09)
Letter from Dr. Sue Bailey, Assistant
Secretary of Defense, Health Affairs, to United States Representative
Alan B. Mollohan (D-WV), explaining DoD policy on jet injectors in
response to the concerns of a constituent of the Congressman. (1998-1999) Manufacturer
discontinuation of large multi-dose vials
for yellow fever, meningococcal, and tetanus-diphtheria vaccines
because of military withdrawal of Ped-O-Jets® capable of using them (MMQC-99-1248
dated 1998-Nov-03 and
MMQC-99-1251 dated 1999-Aug-12). (1998-11-25)
Navy Bureau of Medicine and Surgery authorizes
military use of new disposable-cartridge jet injector (Preventive
Medicine Directorate). Current DoD policies and information available at the Military Immunization Information Source http://www.cdc.gov/nip/dev/jetinject.htm (link no longer active. This is a copy of .pdf file 2004 CDC Jetgun Bio Update )
Admission- Problems Existed With Jet
Injectors
NARR/REF A IS BUMED IMMUNIZATION
REQUIREMENTS AND AUTOMATIC JET
HYPODERMIC INJECTION UNITS/WITHDRAWAL
(DPSC 970147).//
A.
BIOJECTOR 2000
INJECTION
MANAGEMENT
SYSTEM IS
AUTHORIZED FOR
USE IN NAVY AND
MARINE CORPS
ACTIVITES FOR
IMMUNIZATION
ADMINISTRATION
TO SERVICE
MEMBERS AND
OTHER
BENEFICIARIES.
AT THIS TIME, NO
OTHER HYPODERMIC
JET INJECTOR
SYSTEM IS FDA
APPROVED-THIS IS
REQUIRED PRIOR
TO CONSIDERATION
FOR BUMED
AUTHORIZATION.
|
|
|
|
|