Safety of Injections
World Health Organization, Geneva, 1998
GLOBAL PROGRAMME FOR VACCINES AND IMMUNIZATION
EXPANDED PROGRAMME ON IMMUNIZATION LL
The Global Programme for Vaccines and Immunization
thanks the donors whose unspecified financial support in 1997
has made the production of this document possible.
Ordering code: WHO/EPI/LHIS/96.05 Rev.1
Printed : December 1996
Revised: October 1998
This document is available on the Internet
at the GPV Document Centre web-site:
http://www.who.ch/gpv-documents/
Copies may be requested from:
World Health Organization
Global Programme for Vaccines and Immunization
CH-1211 Geneva 27, Switzerland
Fax: +22 791 4193/4192; E-mail: gpv@who.ch
© World Health Organization 1998
This document is not a formal publication of the World Health Organization
(WHO), and all rights are reserved by the Organization. The document may, however, be freely reviewed, abstracted, reproduced and translated, in part or in whole, but not for sale nor for use in conjunction with commercial purposes.
The views expressed in documents by named authors are solely the responsibility of those authors. LLL
Contents
WHO-recommended policy: Safety of injections in
immunization services......................................................................................... 1
1. Selection of injection equipment.............................................................. 2
1.1 Types of equipment................................................................................... 2
1.2 Auto-destruct syringes with fixed needles................................................. 2
1.3 Standard disposable syringes..................................................................... 2
1.4 Sterilizable Syringes .................................................................................. 2
1.5 Needle-free injectors................................................................................. 3
1.6 Safety containers for the disposal of used syringes, needles and sharps .... 3
1.7 Incineration equipment ............................................................................. 3
2. Critical use and disposal procedures......................................................... 4
2.1 Auto-destruct and standard disposable syringes and needles .................... 4
2.2 Sterilizable syringes and needles ............................................................... 4
2.3 Transport and disposal of contaminated sharps ........................................ 5
3. Supervision and evaluation......................................................................... 6
3.1 Supervisory visits ...................................................................................... 6
3.2 Assessment of injection practices .............................................................. 6
3.3 Routine monitoring................................................................................... 6
4. Budgeting and supply................................................................................... 7
4.1 Disposable injection equipment................................................................. 7
4.2 Reusable injection equipment.................................................................... 7
4.4 Safety containers ....................................................................................... 7
4.4 Distribution system................................................................................... 7
4.5 Advance budget......................................................................................... 7
Annex 1: Critical questions for supervisory checklist to determine
injection safety...................................................................................................... 8
WHO-recommended policy:
Safety of injections
in immunization services
An injection should only be given if it is necessary -- and each injection that is given must be safe.
· An injection for immunization is necessary.
· An immunization injection is safe when the vaccine is injected with the appropriate equipment and according to the recommended procedures for injection, sterilization and disposal.
The proper techniques for immunization injections have been specified in a previous document
(EPI/PHW/84.03 Rev. 1). The scope of this document is, therefore, limited to the selection of injection equipment and the procedures which are critical1 for the safe use of the equipment. This document should be read in conjunction with the WHO-UNICEF policy statement for mass immunization campaigns
(WHO/EPI/LHIS/97.04 Rev.1).
"Critical" in this case refers to the most important of many sterile procedures which are already recommended in
WHO/EPI training documents.
_ 6DIHW\_RI_LQMHFWLRQV_LQ_LPPXQL]DWLRQ_SURJUDPPHV
1. Selection of injection equipment
1.1 Types of equipment
The following equipment can be used to administer injectable vaccines:
· Auto-destruct syringes with fixed needles
· Sterilizable syringes and needles
· Standard disposable syringes and needles
The following additional equipment is necessary to assure safety:
· Safety boxes for the collection of used syringes, needles and sharps
· Incinerators
Each type of equipment is safe only if users follow the critical procedures specified for its use. (See sections 2-4.)
1.2 Auto-destruct syringes with fixed needles
The auto-destruct syringe is the recommended type of disposable equipment for parenteral administration of vaccines. This type of syringe is the equipment of choice for conducting mass immunization. The auto-destruct syringe presents the lowest risk of person to person transmission of bloodborne pathogens because itcannot be reused.
1.3 Standard disposable syringes
Standard disposable syringes and needles should only be used for immunization only in settings where it is guaranteed that they will be destroyed after a single use, as verified by monitoring of consumption and supervision of disposal. WHO warns governments and donor agencies that the reuse of standard disposable syringes and needles places the general public at high risk of disease and death.
1.4 Sterilizable Syringes
Sterilizable syringes can be used for routine immunization sessions where compliance with cleaning and sterilization procedures between each use can be assured, as verified by supervisory visits and by routine use of ‘time, steam & temperature’
(TST) control spots. Sterilizable syringes are neither practical nor economic for mass immunization sessions and should not be used for this purpose.
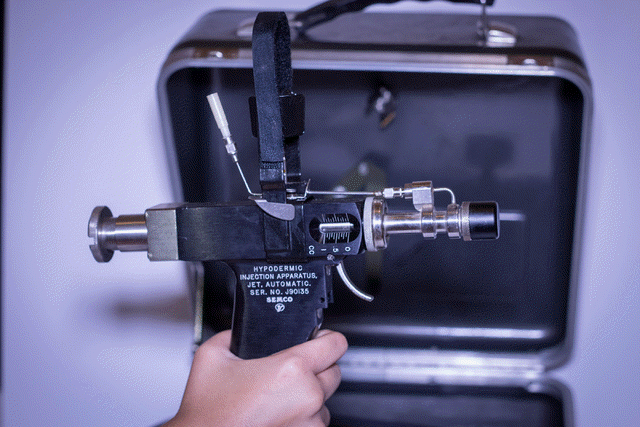
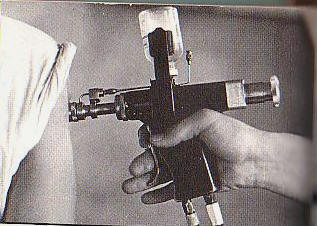
1.5 Needle-free injectors
Needle-free injectors designed for use with multi-dose vials and with a multiple-use fluid path should not be used for immunization. These injectors have an inherent risk of bloodborne disease transmission. Needle-free injectors designed for use with mono-dose pre-filled cartridges, or mono-dose cartridges for filling at the point of use, with a disposable fluid path may be used for immunization. These injectors do not share the same risk of contamination and disease transmission as the multi-dose injectors.
1.6 Safety containers for the disposal of used syringes, needles and
sharps Puncture resistant containers for collecting and disposing of used disposable and auto-destruct syringes, needles and other injection materials must be provided and used in all immunization activities. These containers reduce the risk posed by contaminated needles and syringes to health staff and to the general public.
1.7 Incineration equipment
Auto-combustion type of incinerators which achieve temperatures in excess of 8000 Celsius are preferred to destroy all contaminated sharps, including syringes and needles used for immunization. This equipment ensures the most complete destruction of used sharps while also reducing environmental pollution.
1
More than 120 immunizations per session
2
Confirmed in animal tests: field trials in progress to confirm risks in humans.
3
Currently used in the USA but not yet widely available: cost in use high.
4
Meeting WHO Standard Performance Specification E10/IC.1 or E10/IC.
_ 6DIHW\_RI_LQMHFWLRQV_LQ_LPPXQL]DWLRQ_ SURJUDPPHV
2. Critical use and disposal procedures
2.1 Auto-destruct and standard disposable syringes and needles
A sterile packed syringe and a sterile packed needle must be used for each injection and effectively destroyed according to the following critical procedures:
2.1.1 Immediately after a single use, place each syringe and needle in a Safety
puncture-resistant container. Do not attempt to recap the needle.
2.1.2 Do not use disposable syringes and needles from damaged or punctured
sterile packs, or which have passed the manufacturer’s expiry date.
2.2 Sterilizable syringes and needles A sterile syringe and a sterile needle must be used for each injection. The critical procedures for handling, cleaning, sterilizing and disposing of reusable syringes and needles are outlined below:
2.2.1 Immediately after injection, flush water through the syringe and needle.
Take the syringe apart and drop it and the needle into a bowl of water. After the immunization session, wash all the syringe components in clean water before loading them for sterilization. Use forceps, not fingers, to pick the syringe components and the needle from the water and place them in the sterilizer.
2.2.2 Steam sterilize reusable needles, syringes and forceps at 121°C-126°C for
20 minutes, according to the instructions of the sterilizer manufacturer. Steam
sterilization kills all harmful viruses, bacteria, and spores1, including those that cause abscesses, tetanus, hepatitis B, and HIV.
2.2.3 Approved sterilization indicators (Time, Steam and Temperature : TST
control spots) must be included in each sterilization cycle. Inspect the indicator at the time of use and attach it to the immunization report.
2.2.4 Dispose of syringes which leak, become too stiff to use or have faded
graduations and also dispose of needles which have become blocked, blunted or hooked. Do not attempt to re-sharpen needles. Boiling and other methods of high level disinfection will not destroy certain
spores.
2.3 Transport and disposal of contaminated sharps
2.3.1 The recommended method of disposal of used syringes and needles is
destructive incineration at temperature above 800 degrees Celsius. Where
incinerators are not available, contaminated sharps should be burned in a pit, drum or constructed hearth. The compound in which the incineration takes place must be secured.
2.3.2 Incineration or burning should be conducted as close to the point of use as possible and as soon after the immunization session as practical. Contaminated sharps must be collected and stored in safety puncture-resistant containers which are preferably incinerated with the syringes.
2.3.3 Contaminated sharps should not be transferred from container to
container. If containers are transported by vehicle, the vehicle should be protected against contamination or disinfected before being used for other purposes.
3. Supervision and evaluation
Systematic supervision and periodic evaluation of injection practices are vital to ensure safety.
3.1 Supervisory visits At least twice each year, make supervisory visits to each health
centre, using a checklist which includes a review of injection safety to improve performance (See Annex 1 : Critical questions for supervisory checklist).
3.2 Assessment of injection practices
Include an assessment of safe injection practices, injection equipment and the equipment supply system in every EPI programme review and other evaluation activities.
3.3 Routine monitoring
Routinely monitor and investigate all injection-related adverse events to improve the quality and safety of injections and assist supervisory procedures.
4. Budgeting and supply
An uninterrupted supply of sufficient injection equipment is critical to the safety of immunizations. The measures which should be taken to assure the availability of adequate supplies include the following:
4.1 Disposable injection equipment
At central and intermediate stores, keep a reserve stock of equipment—at least 10% of the quantity used in each supply period. At peripheral stores keep a reserve stock that is sufficient for at least one month of immunization activities.
4.2 Reusable injection equipment
Keep a minimum level of syringes and needles in stock equal to the largest number of injections given at a single session, plus an additional 10% reserve. Provide sufficient fuel for sterilization and spares parts for the maintenance of steam sterilizers.
4.4 Safety containers
Provide safety puncture-resistant containers1 in sufficient quantities to all health units for the collection and incineration of contaminated syringes and needles.
4.4 Distribution system
Establish a distribution system for all injection equipment which is the same as that for vaccines, with the following characteristics:
· a timetable of regular supply dates an estimate of routine needs based on rates of use, planning of needs for special immunization activities, and
· a record of current stock levels.
4.5 Advance budget
One year in advance, establish an adequate budget for the supply of sufficient injection, sterilization and disposal equipment to cover routine immunization, special immunization activities and, if necessary, the restoration of reserve stocks. Constructed according to
WHO/EPI Standard Equipment Specification E10/IC.1.
_ 6DIHW\_RI_LQMHFWLRQV_LQ_LPPXQL]DWLRQ_ SURJUDPPHV