|
|
||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||
|
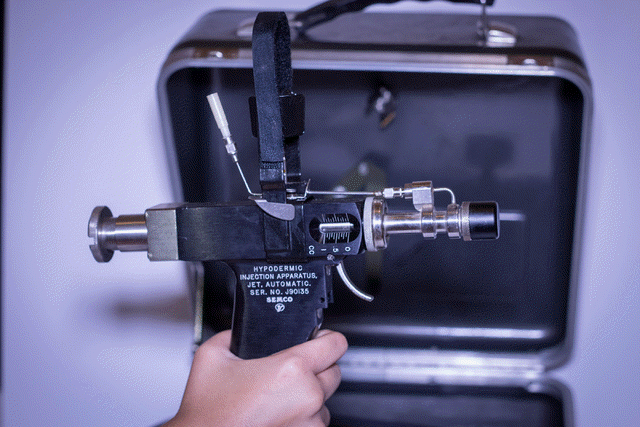
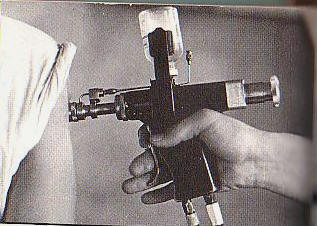
The study ended early because the protector cap needle-free injector (PCNFI) failed to prevent contamination in the first batch tested (8.2% failure rate). Vaccine. 2008 Mar 4;26(10):1344-52. Epub 2008 Jan 18. Preventing contamination between injections with multiple-use nozzle needle-free injectors: a safety trial. PATH, 1455 NW Leary Way, Seattle, WA 98107, USA. kkelly@path.org Multiple-use nozzle jet injectors (MUNJIs), a type of needle-free injector, use a high-pressure stream to penetrate skin and deliver medicament. Concerns for their potential to transmit blood borne pathogens led to development of a hybrid MUNJI for use in mass immunizations. The HSI-500, referred to here as a protector cap needle-free injector (PCNFI), utilizes a disposable cap as a shield between the reusable injector nozzle and the skin to reduce the risk of contamination. This study aimed to determine the presence of hepatitis B virus (HBV) contamination in post-injection ("next person") samples immediately following injection in HBV-carrier adults. Tolerability and pain were also assessed. The study ended early because the PCNFI failed to prevent contamination in the first batch tested (8.2% failure rate). The injections were very well tolerated, with most followed by no bleeding (81.2%) or mild bleeding (7.8%). 55.2% of participants experienced no pain while 42.3% experienced mild pain following injection. PMID: 18272265 [PubMed - indexed for MEDLINE]
|
|
|
|