|
|
||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||
|
From Journal of Viral Hepatitis Management of Chronic Hepatitis C Patients Who have Relapsed or Not Responded to Pegylated Interferon Alfa Plus Ribavirin
Read this article on Medscape's
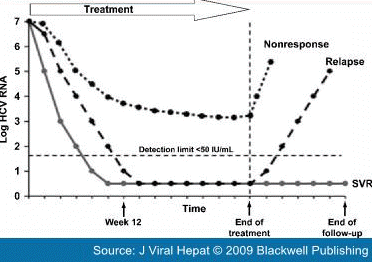
free mobile app.Download Now Abstract and IntroductionAbstractDevelopment of therapeutic strategies for patients with chronic hepatitis C who experience virological breakthrough, relapse or nonresponse lag behind those for treatment-naïve patients. The probability of a previously treated patient responding to re-treatment depends on the nature of the previous regimen, the magnitude of the response to previous treatment and the patient's characteristics. Relapsers have higher sustained virological response rates than nonresponders when re-treated with pegylated interferon plus ribavirin. Re-treatment of nonresponders to pegylated interferon plus ribavirin with the standard 48-week regimen resulted in an approximate 6% sustained response rate in the EPIC-3 program. In the REPEAT trial, the sustained response rate was significantly higher in nonresponders to pegylated interferon alfa-2b (12 kD) plus ribavirin randomized to 72 weeks of peginterferon alfa-2a (40 kD) plus ribavirin, compared with a 48-week regimen (16% vs 8%, P = 0.0006). Based on available data, extended treatment is the best option for these individuals. Undetectable viral RNA at week 12 is an important criterion for re-treatment in the REPEAT and EPIC studies. Maintenance therapy with pegylated interferon is generally ineffective in nonresponders and cannot be recommended. Directly acting antivirals may increase response rates and the burden of adverse events when combined with the standard of care, but will not be available for some years. In conclusion, after careful evaluation of an individual's benefit–risk ratio, a 72-week regimen is the preferred strategy for optimizing sustained response rates in patients who have not responded to the standard of care, provided that viral RNA is undetectable at week 12 of re-treatment. IntroductionInfection with hepatitis C virus (HCV) often results in chronic hepatitis C, a progressive liver disease that is associated with significant morbidity and mortality. Chronic HCV can lead to liver cirrhosis and hepatocellular carcinoma and is one of the leading indications for liver transplantation worldwide. However, effective treatment is available. The current standard of care for chronic HCV infection is the combination of pegylated interferon plus ribavirin.[1] With this combination, more than 50% of previously untreated patients achieve a sustained virological response (SVR).[2–5] SVR has been described as 'tantamount to a cure' by the American Gastroenterological Association.[6] Cure of chronic hepatitis C is associated with resolution of symptoms, improvements in health-related quality of life and liver histology and reductions in liver-related morbidity and mortality.[7–14] Cure rates for chronic hepatitis C have improved significantly with each major evolutionary step in the treatment paradigm for the disease. As the willingness to treat patients and the indications for treatment have expanded, so too have the number of patients in whom HCV has not been eradicated by a first course of treatment. Thus, there is a large and growing pool of 'relapsers' and 'nonresponders' to the standard of care in urgent need of effective treatment. Much progress has been made in optimizing treatment regimens for patients with chronic hepatitis C.[15,16] However, the development of therapeutic strategies for relapsers and nonresponders lags behind those for treatment-naïve patients. As a result, current treatment guidelines recommend that re-treatment should only be contemplated if the previous course of treatment included conventional (i.e. nonpegylated) interferon.[1] This recommendation is now in need of re-evaluation as recent data have provided insight into how best to re-treat patients with pegylated interferon plus ribavirin. Definition of Relapse and NonresponseRelapse and nonresponse are defined on the basis of the virological response to treatment (Fig. 1). A patient is said to have experienced a virological relapse if HCV RNA decreases and remains below the limit of detection (<50 IU/mL) during treatment but becomes detectable after cessation of treatment. If HCV RNA rebounds and becomes detectable in such a patient before treatment is completed, this is referred to as virological breakthrough. Virological nonresponse is evident when the serum HCV RNA level remains above the limit of detection throughout treatment and is formally defined as less than 2 log10 decline in HCV RNA between baseline and week 12. A patient who has less than 1 log10 reduction in serum HCV RNA at week 12 of treatment is said to have had a null response.
A panel of experts convened by the US Food and Drug Administration recently issued a position statement that included a formal definition of nonresponse for use in future clinical trials (Table 1).[17] In contrast to the situation in clinical trials, in everyday clinical practice, the extent of previous treatment (i.e. dose and duration) and the magnitude of the virological response are often unknown.
Which Patients are Most Likely to Relapse or Not Respond to the Standard of Care?HCV has evolved such that it impairs innate and adaptive host immune mechanisms.[18] However, at present, it is not possible for clinicians to identify nonresponders to treatment with pegylated interferon before treatment is initiated. Certain factors, including infection with HCV genotype 1, high baseline HCV RNA level, African American race, advanced hepatic fibrosis, obesity and insulin resistance, are associated with lower SVR rates, but the presence of one or more of these factors does not preclude a successful treatment outcome. Recent genetic studies have provided insight into the biological basis of nonresponse to interferon-based therapies,[19] and in the future, this may make it possible to differentiate between potential nonresponders and responders to treatment on the basis of gene expression patterns in liver tissue obtained before treatment is initiated.[20–23] In the current standard of care for treatment-naïve patients, on-treatment virological responses at weeks 4 and 12 have evolved as important predictive tools to determine the chance of success. The later a patient clears HCV RNA from the blood, the higher the probability of relapse or nonresponse.
|
|
|
|